Day 2 :
Keynote Forum
Claudio Santi
University of Perugia, Italy
Keynote: New drugs and catalysts inspired by glutathione peroxidase
Time : 10:00-10:30

Biography:
Claudio Santi received the PhD in Chemical Sciences from the University of Perugia under the supervision of Prof. Marcello Tiecco. Currently he is Professor of Organic Chemistry and he leads the group of Catalysis and Organic Green Chemistry in the Department of Pharmaceutical Sciences. He is worldwide recognized expert n organoselenium chemistry. His research interest range from the application of selenium reagents in green chemistry to the development of new orgaselenium containing drugs. He is author of more than 130 publications including review articles and book chapters.
Abstract:
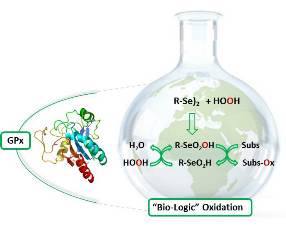
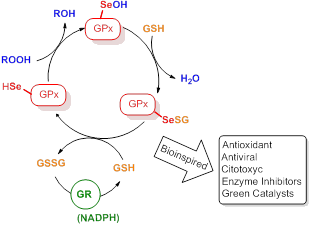
Glutathione Peroxidase (GPx) among the currently known selenoenzymes, is the best characterized in term of chemical structure and reaction mechanism. The catalytic center of this enzyme is a selenocysteine and, more specifically, a selenium atom that is stabilized by a catalytic triad in the form of nucleophilic selenate. In this form the selenium is reactive toward peroxides determining their reduction into the non-harmful alcohol or water. The selenol by reaction with the peroxide is transformed into the corresponding selenenic acid that is rapidly reduced by 2 molecules of glutathione affording a molecule of oxidized glutathione and the native selenate that is ready to start a second cycle. Glutathione peroxidase have a crucial role into control and prevent the damage produced by the reactive oxygen species (ROS) in living system and, from one side it is important to maintain a healthy status from the other it is necessary to reinforce it during a number of pathologic situation. During the last decades, several small molecules containing selenium as well as some artificial selenoenzymes were developed and tested as antioxidant but also as prooxidant as enzyme inhibitors, hormetic agents, antiviral, anticancer, antimicrobial. In this talk I’ll report the state of art of the research on this field focusing some new prospective that are currently ongoing in our laboratory: discovery of new biologically active organoselenium compounds and determination of their reaction mechanism in living systems. Beside that the bio inspiration is an excellent strategy for the development of new efficient and eco sustainable catalyst for application in Green Chemistry, some recent example of these reults will be presented and discussed.
References:
- Sancineto, L., Piccioni, M., De Marco, S., Pagiotti, R., Nascimento, V., Braga, A.L., Santi, C., Pietrella, D. Diphenyl diselenide derivatives inhibit microbial biofilm formation involved in wound infection (2016) BMC Microbiology, 16 (1), art. no. 220H
- Sancineto, L., Mariotti, A., Bagnoli, L., Marini, F., Desantis, J., Iraci, N., Santi, C., Pannecouque, C., Tabarrini, O. Design and Synthesis of DiselenoBisBenzamides (DISeBAs) as Nucleocapsid Protein 7 (NCp7) Inhibitors with anti-HIV Activity (2015) Journal of Medicinal Chemistry, 58 (24), pp. 9601-9614
- Bartolini, D., Commodi, J., Piroddi, M., Incipini, L., Sancineto, L., Santi, C., Galli, F. Glutathione S-transferase pi expression regulates the Nrf2-dependent response to hormetic diselenides (2015) Free Radical Biology and Medicine, 88 (Part B), pp. 466-480.
- Bartolini, D., Piroddi, M., Tidei, C., Giovagnoli, S., Pietrella, D., Manevich, Y., Tew, K.D., Giustarini, D., Rossi, R., Townsend, D.M., Santi, C., Galli, F. Reaction kinetics and targeting to cellular glutathione S-transferase of the glutathione peroxidase mimetic PhSeZnCl and its d,l-polylactide microparticle formulation (2015) Free Radical Biology and Medicine, 78, pp. 56-65.
- Santoro, S., Azeredo, J.B., Nascimento, V., Sancineto, L., Braga, A.L., Santi, C. The green side of the moon: Ecofriendly aspects of organoselenium chemistry (2014) RSC Advances, 4 (60), pp. 31521-31535..
Keynote Forum
Toshiyuki Moriuchi
Osaka University, Japan
Keynote: Bromoperoxidase mimicking bromination catalysts
Time : 10:50-11:20

Biography:
Toshiyuki Moriuchi received his bachelor’s degree in 1991 and his doctoral degree in 1995 under the supervision of Professor Toshikazu Hirao, both from Osaka University. He became Assistant Professor at Osaka University and was a Postdoctoral fellow at California Institute of Technology with Professor Jacqueline K. Barton (1996–1997). Dr. Moriuchi was promoted to Associate Professor in 2004. His current research interests focus on the development of novel artificial bioconjugated systems based on self-organization of biomolecules and redox-active ï°-conjugated systems for functionalized catalysts and materials. He received the Inoue Research Award for Young Scientists in 1997 and HGCS Japan Award of Excellence 2011 in 2012.
Abstract:
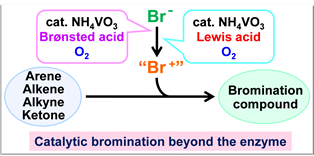
Haloperoxidases are enzymes that are able to catalyze the oxidation of halide ions by using hydrogen peroxide. Catalytic activities of haloperoxidases have received great attention because of their capability to halogenate a variety of organic compounds. Vanadium bromoperoxidase (V-BrPO), which is a naturally occurring enzyme found in marine algae, is a kind of haloperoxidase. V-BrPO catalyzes two-electron oxidation of the bromide ion in the presence of hydrogen peroxide, affording a bromonium cation-like species. V-BrPO has been demonstrated to perform the catalytic bromination of organic compounds. Bromination reaction is one of the most fundamental reactions in organic synthesis, providing important precursors and substrates in various coupling reactions. Conventional bromination reaction is performed by using hazardous and toxic elemental bromine. Considerable efforts have been focused on developing a versatile bromination method with a bromide ion as a bromide source instead of bromine. So, the V-BrPO mimicking bromination reaction systems induced by a vanadium catalyst and hydrogen peroxide have attracted much attention. These catalytic systems, however, require a stoichiometric amount of a strong oxidant to generate the bromonium-like species. A more practical catalytic bromination reaction system without use of hazardous reagents is to be developed. From the view point of green chemistry perspective, molecular oxygen is regarded as the best candidate for oxidants. We embarked upon the development of an environmentally-favorable catalytic method for selective bromination of a wide range of substrates. In this presentation, bromoperoxidase mimicking versatile and practical bromination catalytic systems by the combination of a commercially available inexpensive ligand-free vanadium catalyst and a Brønsted acid or a Lewis acid under molecular oxygen will be described.
- Enzymology & Biochemistry | Structural Enzymology
Location: Olimpica 1
Session Introduction
Brian G Miller
Florida State University, USA
Title: Allokairic regulation of enzyme function
Time : 10:50-11:10

Biography:
Brian Miller is an Associate Professor of Biochemistry at the Florida State University, USA. He did his Ph.D. from the University of North Carolina, Chapel Hill in the year 2001. His research interest is protein structure, function and evolution.
Abstract:
Human glucokinase (GCK), the body’s primary glucose sensor and a major determinant of glucose homeostatic diseases, displays a unique form of allosteric-like behavior that is manifested as a cooperative kinetic response to glucose. The allosteric-like behavior of GCK is particularly intriguing since the enzyme is monomeric and contains only one glucose binding site. Recent work in our laboratory has shown that millisecond timescale order-disorder transitions within the enzyme’s small domain govern cooperativity. Here, we present the results of biophysical studies that elucidate the structural and dynamic origins of the time-dependent, “allokairic” properties of GCK. Using high-resolution nuclear magnetic resonance we identify two distinct mechanisms by which GCK can be activated, both of which result in hyperinsulinemia. The first activation mechanism alters the equilibrium distribution of GCK conformers in favor of a single-state, whereas the second mechanism alters the intrinsic dynamics of the enzyme without perturbing the relative distribution of states in the structural ensemble. Time-resolved fluorescence measurements map the dynamic conformational landscape of GCK and provide evidence for three distinct conformations of the enzyme in the absence of glucose. Together our findings provide a framework for understanding the origins of time-dependent changes in activity in other regulatory enzymes.
Min Kyu Kwak
Seoul National University, South Korea
Title: Candida albicans glutathione reductase downregulates Efg1-mediated cyclic AMP/protein kinase-A pathway and leads to defective hyphal growth and virulence upon decreased cellular methylglyoxal content accompanied by activating alcohol dehydrogenase and glycolytic enzymes
Time : 11:10-11:30

Biography:
Min-Kyu Kwak has his expertise in Metabolic Regulation driven by physiological roles of methylglyoxal biosynthesis/degradation enzymes. His model based on methylglyoxal production/detoxification hierarchy suggests different metabolic control systems between prokaryotes and eukaryotes. Because his findings provide a basis for understanding cell growth, viability, and differentiation to elevate the intracellular metabolites including methylglyoxal, glutathione, and reactive oxygen species, this should be of interest to scientists who are interested in the methylglyoxal metabolism and it’s regulating enzymes in cells. His research is also to elucidate the mechanism of energy transfer which is a fundamental mechanism in life. Energy transfer mechanisms are mediated by electrons and photons. He aims to elucidate the function and structure of intermediate products, enzymes and genes involved in cell change down to the level of electrons and photons; mechanisms of “polymorphic changes of Candida albicans, developmental processes of Dictyostelium discoideum, and sporulation of Bacillus subtilis regarding electron transfer.
Abstract:
Glutathione reductase maintains the glutathione level in a reduced state. As previously demonstrated, glutathione is required for cell growth/division and its biosynthesizing-enzyme deficiency causes methylglyoxal accumulation. However, experimental evidences for reciprocal relationships between Cph1-/Efg1-mediated signaling pathway regulation and methylglyoxal production exerted by glutathione reductase on yeast morphology remain unclear. Glutathione reductase (GLR1) disruption/overexpression was performed to investigate aspects of pathological/morphological alterations in Candida albicans. These assumptions were proved by observations of cellular susceptibility to oxidants and thiols, and measurements of methylglyoxal and glutathione content in hyphal-inducing conditions mainly through the activity of GLR1-overexpressing cells. Additionally, the transcriptional/translational levels of bio-energetic enzymes and dimorphism-regulating protein kinases were examined in the strain. The GLR1-deficient strain was non-viable when GLR1 expression under the control of a CaMAL2 promoter was conditionally repressed, despite partial rescue of growth by exogenous thiols. During filamentation, non-growing hyphal GLR1-overexpressing cells exhibited resistance against oxidants and cellular methylglyoxal was significantly decreased, which concomitantly increased expressions of genes encoding energy-generating enzymes, including fructose-1,6-bisphosphate aldolase, glyceraldehyde-3-phosphate dehydrogenase, and alcohol dehydrogenase (ADH1), with remarkable repression of Efg1-signaling cascades. This is the first report that GLR1-triggered Efg1-mediated signal transduction repression strictly reduces dimorphic switching and virulence by maintaining the basal level of methylglyoxal following the enhanced gene expressions of glycolytic enzymes and ADH1. The Efg1 downregulatory mechanism by GLR1 expression has possibilities to involve in other complex network of signal pathways. Understanding how GLR1 overexpression affects multiple signaling pathways can help identify attractive targets for antifungal drugs.

References:
- Kwak M K, Liu R, Kwon J O, Kim M K, Kim A H, Kang S O (2013) Cyclic dipeptides from lactic acid bacteria inhibit proliferation of the influenza A virus. J. Microbiol. 51: 836-843.
- Kwak M K, Liu R, Kim M K, Moon D, Kim A H, Song S H, Kang S O (2014) Cyclic dipeptides from lactic acid bacteria inhibit the proliferation of pathogenic fungi. J. Microbiol. 52: 64-70.
- Kwak M K, Ku M, Kang S O (2014) NAD+-linked alcohol dehydrogenase 1 regulates methylglyoxal concentration in Candida albicans. FEBS Lett. 588(7): 1144-1153.
- Kwak M K, Song S H, Ku M, Kang S O (2015) Candida albicans erythroascorbate peroxidase regulates intracellular methylglyoxal and reactive oxygen species independently of D-erythroascorbic acid. FEBS Lett. 589(15): 1863-1871.
- Kwak M K, Lee M H, Park S J, Shin S M, Liu R, Kang S O (2016) Polyamines regulate cell growth and cellular methylglyoxal in high-glucose medium independently of intracellular glutathione. FEBS Lett. 590(6): 739-749.
Petra Borilova Linhartova
Masaryk University, Czech Republic
Title: Biotechnological and molecular genetics approaches in the study of enzymes involved in the etiopathogenesis of dental caries
Time : 11:30-11:50

Biography:
Petra Borilova Linhartova has completed her Master’s degree at the Faculty of Science and PhD at the Faculty of Medicine Masaryk University Brno, Czech Republic. She is a team member in the project promoting excellence in basic research, Czech Science Foundation Centre of Orofacial development. She has published 12 papers in reputed journals and has been serving as a reviewer of international journals.
Abstract:
Dental caries is a complex chronic multifactorial disease representing a major oral health problem in the world. In its pathogenesis, metabolic activity of cariogenic bacteria and host enzymes involved in the immune response and dentine formation plays an important role. The advanced molecular genetics methods allow by combining the sophisticated cultivation techniques with genome-level studies to investigate more thoroughly how bacterial pathogens respond to environmental stimuli. In vitro study was focused on changes in metabolomes/transcriptomes of cariogenic bacteria (Streptococcus mutans, etc.) in dependence on external conditions, such as various substrates (carbohydrates, human/animal milk, and infant formula). Their cariogenic potential was evaluated by measuring acidity of the environment and biomass concentration. The composition of metabolites and gene expression profiles were monitored by modern biotechnology techniques (CE/HPLC/MS and NGS, respectively). Further, case-control association study comprising 803 Czech children (172 controls and 111/520 patients with dental caries in the primary/permanent dentition) was carried out. Candidate genes encoding matrix metalloproteinase-9 and -20 (MMPs), which are included in the development, remodeling and destruction of oral tissues, were selected for the analysis. Polymorphisms rs17576 and rs1784418 were determined by real-time PCR using TaqMan assays. Both SNPs were associated with severity of but not susceptibility to dental caries in the permanent dentition (P<0.05).Biotechnological and molecular genetics approaches offer new possibilities for the study of complex diseases etiopathogenesis, such as dental caries.
Dejan Bezbradica
University of Belgrade, Serbia
Title: Enzymatic synthesis of prebiotic galacto-oligosaccharides: application of nanobiocatalysts and structural characterization of product
Time : 11:50-12:10

Biography:
Dejan Bezbradica obtained his PhD degree in the Department of Biochemical Engineering and Biotechnology of Faculty of Technology and Metallurgy in Belgrade in 2007. Since 2013, he is an Associate Professor in the Department of Biochemical Engineering and Biotechnology. During 2009, he was on sabbatical working in the Laboratory of Enzyme Engineering at Institute of Catalysis in Madrid. His scientific work covers following areas: Cell and enzyme immobilization, enzymatic synthesis in microaqueous media, application of membrane reactors in biocatalytic processes; microbial production and purification of industrial enzymes, kinetic modeling of bisubstrate enzymatic reactions, application of enzymes with transglycosylative activity in synthesis of bioactive compounds, chemical modification of enzymes and immobilization supports, and nanobiocatalysis. His recent research activities are focused on the development of food and feed products containing bioactive galactosides with prebiotic activities targeted for specific probiotic species.
Abstract:
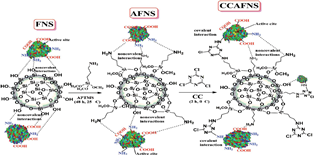
Statement of Problem: Galacto-oligosaccharides (GOS) are group of β- galactoside compounds with significant market value due to their prebiotic properties utilized in infant nutrition products. Physiological activity is based on their short chain carbohydrate structure which makes them non-digestible by digestive enzymes, but digestible by beneficial probiotic bacteria with consequential property of selective promotion of their growth and improvement of overall health status. State of the art in current industrial GOS production based on transgalactosylation activity of β-galactosidases implies that attempts for further advance could be focused on: Fine-tuning of physiological properties by targeted control of enzymatic process toward obtaining GOS of desired structure and developing novel immobilized β-galactosidase preparations with improved affinity towards GOS synthesis.
Methodology & Theoretical Orientation: For evaluation of the effect of enzyme origin on degree of polymerization and type of β-linkages within obtained GOS compounds, transgalactosylation was performed with different β-galactosidases: from Aspergillus oryzae and Lactobacillus acidophilus. Elucidation of chemical structures in obtained GOS mixtures was performed using ion-mobility spectrometry−tandem mass spectrometry (IMS-MS/MS) one-step approach. Improvement in the field of β-galactosidase immobilization was attempted by producing novel nanobiocatalyst with functionalized nonporous fumed nano-silica (FNS) particles as immobilization support.
Conclusion & Significance: IMS-MS/MS analysis has shown that structure of obtained GOS is influenced by origin of β-galactosidase, since one from A. oryzae produced GOSs with β(1→6) and β(1→3) linkages, while enzyme from L. acidophilus produces GOSs with β(1→6) and β(1→4) linkages. Type of glycosidic linkages influences prebiotic properties of GOS, hence determination of linkage type will have great significance in enabling adequate selection of β-galactosidase for targeted prebiotic application. The immobilization on nano-supports indicated that the most adequate support is one functionalized with amino groups, which enabled several times higher transgalactosylation activities than conventionally immobilized β-galactosidase.
References:
- Banjanac K, Mihailović M, Prlainović N, Carević M, Stojanović M, Marinković A, Bezbradica D (2016) Cyanuric chloride functionalized silica nanoparticles for covalent immobilization of lipase. Journal of Chemical Technology and Biotechnology 91: 439-448.
- Banjanac K, Carević M, Ćorović M, Milivojević A, Prlainović N, Marinković A, Bezbradica D (2016) Novel β-galactosidase nanobiocatalyst systems for application in the synthesis of bioactive galactosides. RSC Advances 6:97216-97225.
- Carević M, Ćorović M, Mihailović M, Banjanac K, Milisavljević A, VeliÄković D, Bezbradica D (2016) Galacto-oligosaccharide synthesis using chemically modified β-galactosidase from Aspergillus oryzae immobilized onto macroporous amino resin. International Dairy Journal 54: 50-57.
- Carević M, Bezbradica D, Banjanac K, Milivojević A, Fanuel M, Rogniaux H, Ropartz D, VeliÄković D (2016) Structural elucidation of enzymatically synthesized galactooligosaccharides using ion-mobility spectrometry−tandem mass spectrometry. Journal of Agricultural and Food Chemistry 64: 3609-3615.
- Carević M, VeliÄković D, Stojanović M, Milosavić N, Rogniaux H, Ropartz D, Bezbradica D (2015) Insight in the regioselective enzymatic transgalactosylation of salicin catalyzed by β-galactosidase from Aspergillus oryzae. Process Biochemistry 50: 782-788.
Ayse Ezgi Unlu
Ankara University, Turkey
Title: The effect of natural deep eutectic solvent on laccase catalyzed polycatechin synthesis
Time : 12:10-12:30

Biography:
Ayse Ezgi Unlu has expertise on enzymes, enzymatic reactions, fermentation, protein synthesis, proteomics, enzymatic biopolymers and green solvents. The synthesis of Naproxen, a member of NSAIDs, was the subject of the master thesis using commercial lipase subjected to various pre-treatment strategies that enhanced the activity. Investigation of different parameters on the production of lipase by Candida rugosa and also proteomic analysis of the isoenzymes was another subject of interest. Two important antioxidant enzymes, catalase and superoxide dismutase production by Rhodotorula glutinis was studied comprehensively during PhD thesis. A post-doc research on the synthesis of flavonoids using green solvents was completed.
Abstract:
Catechin is a crucial member of flavonoids that show antioxidant properties both in vivo and in vitro. However, flavonoid monomers, like catechin, have some disadvantages such as low solubility and pro-oxidant activity. These drawbacks are reported to disappear in the polymerized form. The polymerization of catechin was reported using organic solvents to provide solubility in many studies. We present here the effect of natural deep eutectic solvent (NADES) as green solvents on laccase catalyzed polycatechin synthesis. The reaction media contained catechin (5 mg ml-1), acetate buffer (pH=5) and betaine (B)-mannose (M) (5:2, molar amount) at mentioned amounts. The effect of B-M amount (5, 50-90%), laccase concentration (15.6-125 U) and temperature (25-40ºC) were investigated on polycatechin synthesis. The antioxidant activities of the polycatechins were tested in terms of superoxide radical scavenging activity and xanthine/xanthine oxidase activity. Size exclusion chromatography and HPLC analysis were used as analytical methods. According to the results, 5% B-M containing reaction media provided high molecular weight polycatechin that was comparable with acetone containing media. Therefore organic solvent content could be discarded from the reaction. However, handling of the reaction media and recovery of the product were challenging steps at increased NADES content. The conversion rate of catechin was found to increase with increasing laccase amount. Additionally, high laccase concentration (125 U) was found to provide high molecular weight and yield. On the other hand, temperature had no significant effect on polycatechin formation at tested range (25-40ºC). All polycatechins obtained were found to have increased superoxide radical scavenging activity and xanthine/xanthine oxidase inhibitory activity when compared to monomer catechin. This study showed that polycatechin synthesis pathway could be shifted to a green route using NADES.

References:
- Unlu A E, Takac S (2012) Investigation of the simultaneous production of superoxide dismutase and catalase enzymes from Rhodotorula glutinis under different culture conditions. Artif. Cells Blood Substit. Immobil. Biotechnol. 40: 338-344.
Mohammed F. EL-Shiekha
Benha University, Egypt
Title: Biochemical effect of some antioxidant on metabolic changes in experimentally induced tumor in female mice
Time : 12:30-12:50

Biography:
Mohammed has completed his PhD from the Department of Biochemistry, Faculty of Veterinary Medicine, Benha University, Egypt. He is a Faculty Member in the Department of Biochemistry, Faculty of Pharmacy, October 6 University, Egypt. He has published 6 papers in reputed journals.
Abstract:
Biochemical effect of tannic acid and curcumin on female mice experimentally induced Ehrlich ascites carcinoma (EAC) was investigated. This study was carried out on 220, 12-14 weeks old female mice and weighted 25-30 g. Mice were classified into two main large experiments. Experiment 1: Non-tumor bearing mice (NTB) Included 100 of animals and divided into four groups each one comprised 25 mice. Group 1: NTB- control saline treated. Group 2: NTB-treated with curcumin orally (350 mg/kg/day) for 6 weeks. Group 3: NTB-treated with tannic acid orally (160 mg/kg/day) for 6 weeks. Group 4: NTB-treated with curcumin and tannic acid orally at ratio (50%:50%) for 6 weeks. Experiment 2: Tumor bearing (TB) mice. Out of the total 120 animals, were divided into four groups each one comprised of 30 mice. Group 1: TBM-control saline treated. Group 2: TBM-treated with curcumin orally (350 mg/kg/day) for 6 weeks. Group 3: TBM-treated with tannic acid orally (160 mg/kg/day) for 6 weeks. Group 4: TBM-treated with curcumin and tannic acid orally at ratio (50%: 50%) for 6 weeks. Blood samples were collected from all animals groups after 2, 4 and 6 weeks from treatment. Serum were separated and processed directly for glucose, insulin, total cholesterol, triacylglycerol, total protein determination. The obtained results revealed that, a highly significant decrease in serum glucose, total cholesterol, total protein concentration, meanwhile, a highly significant increase in serum triacylglycerol concentration was also observed. But a non-significant decrease in serum insulin levels were observed in tumor bearing mice when compared with control. The results of this study indicated that curcumin, tannic acid and their combination treatment have potential benefits in cancer treatment.
Rachel Chen
Georgia Institute of Technology, USA
Title: Metabolic engineering strategies for effective use of glycosyltransferases in oligosaccharide
Time : 12:50-13:10

Biography:
Rachel Chen received her PhD from California Institute of Technology in 1994 and subsequently worked as a Research Scientist in Bristol-Myers Squibb. She began her independent academic career in Virginia Commonwealth University and continued at Georgia Institute of Technology. Her research interfaces Biology, Chemistry, and Engineering with major focuses on applying molecular engineering tools in the synthesis of molecules that are not attainable with conventional means. She has published over 80 peer-reviewed papers and has been serving as an Associate Editor for Microbial Cell Factories and on Editorial Boards of Biotechnology and Bioengineering, AIMS Bioengineering and AIMS Microbiology.
Abstract:
As one of the four building blocks of life, sugar molecules permeate almost all aspects of life. The widespread occurrence of glycosylation and its broad impact in biological processes underscores the importance of studying glycosylation. To study glycans and probe their roles in a biological system significant amount of pure molecules are needed. Besides basic research, there are a wide range of opportunities of utilizing oligosaccharides, polysaccharides, and glycoproteins and other glyco-conjugates for diagnosis, vaccine development, as new drug entities, and many other medical applications. Unfortunately, these potential applications are all impeded by the lack of large scale synthesis technology for these molecules. Metabolic engineering, since its inception in late 80’s, has grown to be a field impactful in the synthesis of a variety of molecules of commercial and societal importance. Opportunities abound at the interface of glycosciences and metabolic engineering. In fact, all sugar moieties in biological components, small or big, free or bound, are important targets for metabolic engineering. Over the past decades, its use in the synthesis of sugar-containing molecules has gained significance. Glycosidic bond formation catalyzed by glycosyltransferase enzyme is in the center of the synthesis of most glycan structures in nature. Oligosaccharides, polysaccharides and glycoproteins share the commonality that requires glycosyltransferases in their synthesis, differing only in the nature of the acceptors. Therefore, from a metabolic engineering point of view, they share much of the synthesis challenges. These include the high energy demand due to the need for sugar nucleotides as precursors, the complexity of metabolic pathways and regulations involved, and the adequate supply of acceptors when and where the glycosyltransferases are most active. Represented by 2’-fucosyllactose, the success in bringing highly valuable oligosaccharides to commercial production demonstrates the power of metabolic engineering. On the other hand, given the enormous diversity and significant complexity of saccharide-containing structures, a handful of molecules attaining commercial success can only qualify as a promising beginning. In fact, the surface of the gigantic glyco-sphere has barely scratched. Providing scientists with hundreds and thousands of glycans in quantities sufficient to probe their structure and function relationships and supplying clinicians with selective compounds (such as Globo H and heparin in Kg quantities) for clinical studies in a cost effective manner are challenges before metabolic engineers and synthetic biologists. The inherent challenges in complex carbohydrates demands innovative metabolic engineering strategies beyond a simple extension of those used in successful examples. In this presentation, metabolic engineering challenges common to glycosyltransferase-catalyzed synthesis of oligosaccharides are analyzed and successful examples from Chen labs are showcased to emphasize the power of metabolic engineering as an enabling technology.
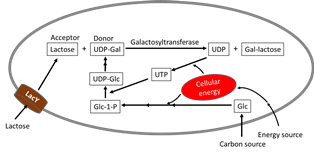
Reference:
- Hyun-Dong Shin, Long Liu, Mi-Kyong Kim, Yong-IL Park, Rachel Chen (2016) Metabolic engineering of Agrobacterium spp. ATCC31749 for curdlan production from cellobiose. J. Ind. Microbiol. Biotechnol. 43: 1323-1331.
- Anne Ruffing and Rachel Chen (2011) Citrate stimulates oligosaccharides synthesis in metabolically engineered Agrobacterium spp. Applied Biochemistry and Biotechnology 164(6):851-66.
- Mao Z, Shin H D, Chen R (2009) A recombinant E. coli bioprocess for hyaluronan synthesis. Applied Microbiology and Biotechnology 84:63-9.
- Anne Ruffing and Rachel Chen (2010) Metabolic engineering of Agrobacterium spp. strain ATCC31749 for production of an alpha-Gal epitope synthesis. Microbial Cell Factories 9:1.
- Anne Ruffing, Zichao Mao, Rachel Chen, (2006) Metabolic engineering of Agrobacterium spp. for UDP-galactose regeneration and oligosaccharide synthesis. Metabolic Engineering 8:465-473.
Valentina Citro
University of Naples Federico II, Italy
Title: Pharmacological chaperones for curing enzymopathies: the case of lysosomal alpha-galactosidase

Biography:
Valentina Citro is interested in developing Pharmacological Chaperones (PC) to cure rare diseases. She works on the identification of the mutations which can be responsive to chaperones and develop method for assays in vitro in two model systems: The Fabry disease, a lysosomal storage disorder and PMM2-CDG (CDG-Ia) disease, a disorder of glycosylation with no cure at present.
Abstract:
Pharmacological chaperones are useful for the treatment of enzymopathies arising from mutations that lower the free energy difference between an unfolded and a folded enzyme shifting the equilibrium towards the first form. The unfolded enzyme, although retaining the functional chemical groups needed for the biological activity, does not maintain them in the appropriate spatial disposition defined as native state. Improperly folded mutant enzymes are usually sensitive to proteolysis and are cleared by the protein quality control systems in the cytosol and endoplasmic reticulum. Activity can be rescued if the equilibrium is pushed back towards the native state. This can be obtained binding a pharmacological chaperone to the folded enzyme. In fact the binding energy of the ligand compensates for the loss in DeltaG of unfolding. Lysosomal alpha-galactosidase represents a good model system for the therapy with pharmacological chaperones. Lysosomal alpha-galactosidase catalyzes the removal of α-galactosyl residues from a glycosphingolipid, globotriaosylceramide. Mutations of lysosomal alpha-galactosidase cause Fabry disease. We use three methods to test the effect of pharmacological chaperones: 1) Thermal shift assay. This test takes advantage of an environmentally sensitive fluorescent dye which binds the enzyme when it reaches the melting temperature; 2) Urea induced unfolding coupled with limited proteolysis and Western blot detection. This test can be carried out on mutants in cell extracts; and 3) Administration of the pharmacological chaperone to cells expressing mutant enzymes. Open reading frames encoding mutated enzymes are introduced into vectors suitable for transient expression. Eukaryotic cells, COS7 or HEK293, are transfected and cultivated in the presence and in the absence of the drug. If the chaperone works and the mutant are stabilized, a larger amount of protein is detected by western blot and consequently a higher enzymatic activity measured.
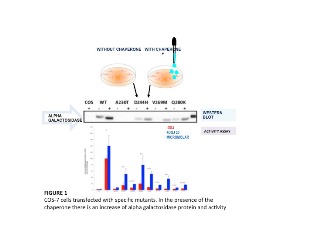
References:
- Andreotti G, Citro V, Correra A, Cubellis M V (2014) A thermodynamic assay to test pharmacological chaperones for Fabry disease. Biochim. Biophys. Acta. 1840: 1214-1224.
- Andreotti G, Citro V, De Crescenzo A, Orlando P, Cammisa M, Correra A, Cubellis M V (2011) Therapy of Fabry disease with pharmacological chaperones: From in silico predictions to in vitro tests. Orphanet J. Rare Dis. 6: 66.
- Citro V, Cammisa M, Liguori L, Cimmaruta C, Lukas J, Cubellis M V, Andreotti G (2016) The large phenotypic spectrum of Fabry disease requires graduated diagnosis and personalized therapy: A meta-analysis can help to differentiate missense mutations. Int J. Mol. Sci. 17.
- Citro V, Pena-Garcia J, den-Haan H, Perez-Sanchez H, Del Prete R, Liguori L, Cimmaruta C, Lukas J, Cubellis M V, Andreotti G (2016b) Identification of an allosteric binding site on human lysosomal alpha-galactosidase opens the way to new pharmacological chaperones for Fabry disease. PLoS One 11: e0165463.
Giuseppe Manfroni
Università degli Studi di Perugia, Italy
Title: Inhibition of the RNA-dependent RNA polymerasic activity of Flavivirus NS5 by heterocyclic compounds
Time : 14:50-15:10

Biography:
Giuseppe Manfroni has graduated in Pharmaceutical Chemistry and Technology (2001) and received his PhD in Medicinal Chemistry (2006) from the University of Perugia (Italy). From 2006 to 2008, he worked as a Post-doctoral Researcher at the University of Perugia. From 2008 to date he is an Assistant Professor in the Department of Pharmaceutical Sciences and is a Lecturer in Pharmaceutical Analysis. He has spent short periods as a Visiting PhD Student at Rega Institute for Medical Research (Leuven, Belgium) and at the Molecular Modeling Laboratory (University of Perugia) under the supervision of Professor Johan Neyts and Professor Gabriele Cruciani, respectively. He is the author of 40 papers, and his research is mainly focused on Medicinal Chemistry of antiviral (HIV, HCV, and Flavivirus), antitumor, and anti-inflammatory (p38 inhibitors) agents. He is an expert on the synthesis of heterocyclic compounds and microwave assisted synthesis.
Abstract:
Among more than 70 related members of Flavivirus genus, Dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus (JEV), Yellow fever virus (YFV), and Zika virus (ZV) are considered (re)-emerging pathogens that were originally endemic in the tropical regions but recently are spreading also in a wider geographic area. Indeed, there are several environmental, demographic, and ecological factors that promote the worldwide diffusion of known and/or novel flaviviruses. Flaviviruses can produce from mild flu-like symptoms to hemorrhagic fevers, hepatitis and neuropathies, such as encephalopathy, meningitis, and microcephaly in human embryos depending on the infective agents. Vaccines are available against YFV, JEV, TBEV, and more recently against DENV but the coverage is far from being complete. Moreover, the lack of an effective and specific therapy further worsens the scenario. The RNA-dependent RNA polymerase (RdRp) of the non-structural NS5 protein is one of the most favored target to find new potential anti- Flavivirus drugs. With the aim to find new inhibitors of the RdRp we undertook a research program exploiting, consecutively, two different approaches: i) A virtual screening carried out on the NS5 polymerase domain (DENV RdRp, 2j7U) followed by a biochemical validation on the isolate target, ii) a direct biochemical screening carried out on DENV NS5 polymerase with the intent to not exclude any potential hit compounds eventually missed during the in silico procedures. Both these approaches were realized using an in-house library of about 200, published and unpublished, compounds previously designed and synthesized as HCV NS5B inhibitors. To validate the potential of the identified hits an anti-viral activity against a panel of Flavivirus was evaluated. The two strategies led us to identify new RdRp inhibitors able to reduce the polymerase activity in the low micromolar range. In particular, the in silico procedure (i) was fruitful for the identification of a pyridobenzothiazole which was extensively characterized with biochemical and structural studies; the second approach (ii) led us to identify functionalized 2,1-benzothiaziens with promising anti-RdRp activity, not emerged as hit compounds during the in silico studies (Figure 1). Also in this case, a representative compound derived from a chemical optimization was better characterized in biochemical and virological assays. The strategy applied in this study led us to identify new promising inhibitors of the NS5 polymerase, worthy of further optimization with the final aim to discover anti-Flavivirus agents.
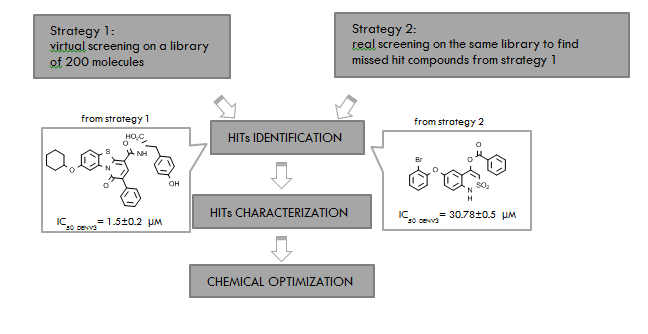
Zeina Nasr
University of Balamand, Lebanon
Title: Knockdown of RPS3, a DNA repair endonuclease, impedes colon cancer growth and progression by decreasing lactate dehydrogenase activity

Biography:
Zeina Nasr passion is to understand the molecular aspect of tumor initiation and progression. Her research focuses on studying the effect of translation initiation dysregulation on cancer behavior. She has worked with several cell lines and transgenic mouse models and deciphered important pathways that contribute to cancer initiation and progression to metastasis. She built her experience by conducting research and teaching in academic institutions. She is currently interested in understanding the extra-ribosomal functions of ribosomal proteins and their effects on tumorigenesis.
Abstract:
Statement of the Problem: In addition to their role in ribosome biogenesis, ribosomal proteins (RPs) play important roles in DNA repair, proliferation, apoptosis and resistance to drugs and chemotherapy. Ribosomal protein S3 (RPS3), a DNA repair endonuclease, is known to be overexpressed in colon adenocarcinoma. In order to ensure their survival, cancer cells rely on aerobic glycolysis catalyzed by the enzyme lactate dehydrogenase (LDH). Our aim is to identify the role of RPS3 in colon cancer growth and metabolism.
Methodology & Theoretical Orientation: Human colon adenocarcinoma Caco-2 and normal colon NCM-640 cells were tested for the expression of RPS3 by Western blot. In order to inhibit RPS3 expression, cells were transfected with siRNA against RPS3 or a non-targeting siRNA (siNT) as a negative control. Upon RPS3 knockdown, cell behaviors were tested including proliferation and survival by trypan blue and WST-1 assays, and cell migration and invasion by the Boyden chamber assays. The glycolysis state of colon cancer cells was assessed by measuring LDH activity upon RPS3 knockdown using the LDH assay.
Findings: RPS3 was shown to be expressed in both Caco-2 and NCM-640 cells. RPS3 knockdown in Caco-2 significantly reduced cell proliferation, survival, migration and invasion compared to siNT-transfected cells. In NCM-640, RPS3 knockdown did not significantly affect cell proliferation and survival implying that RPS3 expression is selectively crucial for colon cancer cell growth. Interestingly, LDH activity was suppressed upon RPS3 knockdown, suggesting a decrease in glycolysis which explains in part the decrease in proliferation.
Conclusion & Significance: This is the first report that shows a role of RPS3 in regulating LDH activity therefore affecting the glycolytic state, the survival and proliferation of cancer cells. Our results also demonstrate that RPS3 is a selective molecular marker in colon cancer and a potential attractive target for colon cancer therapy.
References:
- Rached J, Nasr Z, Abdallah J, Abou-Antoun T (2016) L1-CAM knockdown radiosensitizes neuroblastoma IMR-32 cells by simultaneously decreasing MycN, but increasing PTEN protein expression. Journal of Oncology 49
- Dow L E, Nasr Z, Saborowski M, Ebbesen S H, Manchado E, Tasdemir N, Lee T, Pelletier J, Lowe S W (2014) Conditional reverse transactivator mouse strains for the efficient induction of TRE-regulated transgenes in mice. PLoS One 9
- Nasr Z, Dow L E, Paquet M, Chu J, Ravindar K, Somaiah R, Deslongchamps P, Porco J A Jr, Lowe S W and Pelletier J (2013) Suppression of eukaryotic initiation factor 4E prevents chemotherapy- induced alopecia. BMC Pharmacol. Toxicol. 14
- Nasr Z, Robert F, Porco J A Jr., Muller W J and Pelletier J (2013) eIF4F suppression in breast cancer affects maintenance and progression. Oncogene 32:861-871.
- Nasr Z and Pelletier J (2012) Tumor progression and metastasis: Role of translational deregulation. Anticancer Research 32:3077-3084.
- Enzymology in Drug Discovery | Clinical Enzymology
Location: Olimpica 1
Session Introduction
Wael M. Rabeh
New York University Abu Dhabi, UAE
Title: The catalytic and structural roles of the Human Hexokinase 2 in cancer
Time : 14:00-14:20

Biography:
Wael Rabeh received his PhD in Biochemistry in the lab of Prof Paul F. Cook where he characterized the last enzymatic reaction of the cysteine biosynthetic pathway in Salmonella typhimurium. In 2005, Dr. Rabeh joined the Structural Genomic Consortium (SGC) at the University of Toronto as a post-doctoral fellow, where he characterized the 3D structure of human proteins with medical relevance using X-ray crystallography. In 2007, Dr. Rabeh joined the lab of Dr. Gergely Lukacs at McGill University for the characterization of a membrane channel that is the main cause of Cystic Fibrosis. Dr. Rabeh’s research is devoted for the characterization of proteins’ structure and mechanism to understand their biological function. Major area of his research focus is devoted for the characterization of disease-causing mechanism of proteins with medical relevance to assist in the discovery and design of new therapeutics using proteins’ structural information and computer simulation.
Abstract:
Glucose metabolism is 200 times higher in cancer in comparison to normal tissue as a strategy to support tumor growth and progression, historically known as the ‘Warburg effect’. Hexokinase is the first enzyme of the glycolytic pathway that catalyzes the phosphorylation of glucose for its activation to glucose-6-phosphate and uses ATP as high-energy source of phosphates. Four isozymes are present in human with Hexokinase 2 (HK2) is most active and specifically expressed in verity of different cancers. However, HK2 binding to the outer mitochondrial membrane not only gives it prime access to ATP generated by the mitochondrial but inhibit apoptosis. Here, we aim to biochemically and structurally characterize interactions of HK2 with the mitochondria and the N-terminal role in catalysis and stability of the full-length enzyme. Here, we solved the crystal structure of human HK2 in complex with glucose and glucose-6-phosphate (PDB code: 2NZT), where it is a homodimer with catalytically active N- and C-terminal domains linked by a seven-turn α-helix. Different from the inactive N-terminal domains of isozymes 1 and 3, the N- domain of HK2 not only capable to catalyze a reaction but it is responsible for thermodynamic stabilizes of full-length enzyme. Deletion of first α-helix of the N-domain that binds to the mitochondria altered the stability and catalytic activity of the full-length HK2. In addition, we found the linker helix between the N- and C-terminal domains to play an important role in controlling the catalytic activity of the N-terminal domain. HK2 is a major step in the regulation of glucose metabolism in cancer making it an ideal target for the development of new anticancer therapeutics. Characterizing the structural and molecular mechanisms of human HK2 and its role in cancer metabolism will accelerate the design and development of new cancer therapeutics that are safe and cancer specific.
Giuseppe Manfroni
Università degli Studi di Perugia, Italy
Title: Inhibition of the RNA-dependent RNA polymerasic activity of Flavivirus NS5 by heterocyclic compounds
Time : 14:20-14:40

Biography:
Giuseppe Manfroni has graduated in Pharmaceutical Chemistry and Technology (2001) and received his PhD in Medicinal Chemistry (2006) from the University of Perugia (Italy). From 2006 to 2008, he worked as a Post-doctoral Researcher at the University of Perugia. From 2008 to date he is an Assistant Professor in the Department of Pharmaceutical Sciences and is a Lecturer in Pharmaceutical Analysis. He has spent short periods as a Visiting PhD Student at Rega Institute for Medical Research (Leuven, Belgium) and at the Molecular Modeling Laboratory (University of Perugia) under the supervision of Professor Johan Neyts and Professor Gabriele Cruciani, respectively. He is the author of 40 papers, and his research is mainly focused on Medicinal Chemistry of antiviral (HIV, HCV, and Flavivirus), antitumor, and anti-inflammatory (p38 inhibitors) agents. He is an expert on the synthesis of heterocyclic compounds and microwave assisted synthesis.
Abstract:
Among more than 70 related members of Flavivirus genus, Dengue virus (DENV), West Nile virus (WNV), Japanese encephalitis virus (JEV), Yellow fever virus (YFV), and Zika virus (ZV) are considered (re)-emerging pathogens that were originally endemic in the tropical regions but recently are spreading also in a wider geographic area. Indeed, there are several environmental, demographic, and ecological factors that promote the worldwide diffusion of known and/or novel flaviviruses. Flaviviruses can produce from mild flu-like symptoms to hemorrhagic fevers, hepatitis and neuropathies, such as encephalopathy, meningitis, and microcephaly in human embryos depending on the infective agents. Vaccines are available against YFV, JEV, TBEV, and more recently against DENV but the coverage is far from being complete. Moreover, the lack of an effective and specific therapy further worsens the scenario. The RNA-dependent RNA polymerase (RdRp) of the non-structural NS5 protein is one of the most favored target to find new potential anti- Flavivirus drugs. With the aim to find new inhibitors of the RdRp we undertook a research program exploiting, consecutively, two different approaches: i) A virtual screening carried out on the NS5 polymerase domain (DENV RdRp, 2j7U) followed by a biochemical validation on the isolate target, ii) a direct biochemical screening carried out on DENV NS5 polymerase with the intent to not exclude any potential hit compounds eventually missed during the in silico procedures. Both these approaches were realized using an in-house library of about 200, published and unpublished, compounds previously designed and synthesized as HCV NS5B inhibitors. To validate the potential of the identified hits an anti-viral activity against a panel of Flavivirus was evaluated. The two strategies led us to identify new RdRp inhibitors able to reduce the polymerase activity in the low micromolar range. In particular, the in silico procedure (i) was fruitful for the identification of a pyridobenzothiazole which was extensively characterized with biochemical and structural studies; the second approach (ii) led us to identify functionalized 2,1-benzothiaziens with promising anti-RdRp activity, not emerged as hit compounds during the in silico studies (Figure 1). Also in this case, a representative compound derived from a chemical optimization was better characterized in biochemical and virological assays. The strategy applied in this study led us to identify new promising inhibitors of the NS5 polymerase, worthy of further optimization with the final aim to discover anti-Flavivirus agents.
Anna Antecka
Lodz University of Technology, Poland
Title: Upstream and downstream processing of fungal laccase
Time : 14:40-15:00

Biography:
Anna Antecka has received her PhD in Environmental Engineering in 2008 from the Lodz University of Technology in Poland, within the Faculty of Process and Environmental Engineering, where she is currently an Assistant Professor in the Department of Bioprocess Engineering. From 2004-2005, she made a one-year scientific stay at the International Institute Zittau, Germany, in the Department of Environmental Biotechnology. In 2002, she studied at the University of Dortmund, Germany. Her main research interests are in microbial ecology and biotechnology of fungal enzymes especially laccase, including its production, purification and characterization as well as enzyme applications for industrial and environmental purposes. Currently, she is focused on projects in the area of integrated continuous up- and downstream processes for the biosynthesis and purification of fungal laccases.a
Abstract:
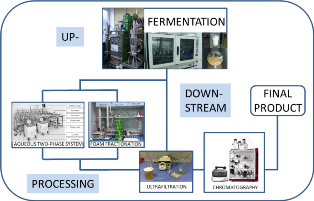
Statement of the Problem: Laccase (EC 1.10.3.2, polyphenol oxidases) belongs to the group of oxidoreductases which is characterized by its specific catalytic properties and the ability to oxidize various organic compounds. Therefore the enzyme is very attractive for a wide range of industrial and environmental purposes. However, due to relatively low effectiveness and the possibility of gradual degradation of bioproducts in the reactor or during the separation and purification stages, there is a need for new approaches and research in this field. Therefore, the purpose of this research was to study and integrate the stages of up- and downstream processing (biosynthesis and purification) of laccase from Cerrena unicolor in order to obtain a highly active enzymatic product.
Methodology: The biosynthesis was performed in a 14-L bioreactor equipped with a set of sensors for process control. Modifications to the medium- addition of microparticles, MPEC, as well as various types of cultivation/growth strategies were examined. The supernatant was concentrated and purified by an aqueous two-phase system (ATPS) consisting of polyethylene glycol and phosphate buffer solutions, and through foam fractionation (FF) at different pH values and with the addition of different detergents. Ultrafiltration and chromatography methods were also investigated. Molecular mass and isoelectric point was determined with the use of electrophoresis.
Findings: Laccase activity increased 3.5-fold after addition of microparticles to the culture media. The fed-batch mode resulted in high laccase activity (up to 4 U/mL) which remains stable during cultivation. The optimal conditions for laccase purification by FF and ATPS were determined with activity partitioning coefficients between foamate and retentate of almost 200 and 2000, respectively, and with yields reaching 50% and 90%, respectively.
Conclusions: Application of MPEC and fed-batch mode proved successful in increasing enzyme production. Both ATPS and FF are successfully applicable for laccase purification.
Jennifer A Littlechild
University of Exeter, UK
Title: Thermophilic enzymes as industrial biocatalysts
Time : 15:20-15:40

Biography:
Jennifer A Littlechild is Professor of Biological Chemistry and has established the Henry Wellcome Centre for Biocatalysis at Exeter University in 2003. Her research studies involve the structural and mechanistic characterisation of a range of enzymes from thermophilic bacteria and Archaea that have industrial applications. She has a particular interest in thermophilic carbonic anhydrase enzymes and has carried out a project with Statoil from 2011-2013. She has published over 200 publications in refereed high impact journals and presented her research work internationally. She is the UK Representative and Vice-chair of the European Section of Applied Biocatalysis and Member of EU advisory committees for industrial biotechnology.
Abstract:
There is an increasing demand for new enzymes with enhanced performance and/or novel functionalities that provide savings in time, money and energy for industrial processes in the areas of high value chemical production and other "white" biotechnology applications. There is a limited understanding of the metabolic capacity of life and only a small proportion of nature’s catalysts have been utilised for industrial biotechnology. There are new metabolic pathways and enzyme activities to be discovered and many of which could be identified within the large proportion of micro-organisms that cannot be cultured and within their associated viruses. The number of enzymes explored to date remains within the range of 1-2% of known microbial diversity. Enzymes used for commercial biotransformation reactions are required to be stable under the industrial conditions employed. The use of naturally thermostable enzymes isolated from hot environments can be a source of enzymes that are more stable to high temperatures, extremes of pH and exposure to organic solvents. By using both genomic and metagenomic approach within the projects, HOTZYME and THERMOGENE, we have identified hydrolase and transferase enzymes of industrial interest isolated from high temperature environments around the world. A selection of these novel enzymes including esterases, cellulases, epoxide hydrolases, transketolases and transaminases have been characterized both biochemically and structurally. In the case of the epoxide hydrolases, two new enzymes with interesting substrate specificity and stereo-selectivity have been discovered from thermophilic metagenomes. Applications of these new epoxide hydrolases have been demonstrated at industrial scale for the production of new chiral chemical building blocks. A new thermophilic cellulase enzyme with activity at pH 5.0 and active under high salt conditions has been isolated which has potential applications for breakdown of biomass.
Valentina Citro
University of Naples Federico II, Italy
Title: Pharmacological chaperones for curing enzymopathies: the case of lysosomal alpha-galactosidase
Time : 15:40-16:00

Biography:
Valentina Citro is interested in developing Pharmacological Chaperones (PC) to cure rare diseases. She works on the identification of the mutations which can be responsive to chaperones and develop method for assays in vitro in two model systems: The Fabry disease, a lysosomal storage disorder and PMM2-CDG (CDG-Ia) disease, a disorder of glycosylation with no cure at present.
Abstract:
Pharmacological chaperones are useful for the treatment of enzymopathies arising from mutations that lower the free energy difference between an unfolded and a folded enzyme shifting the equilibrium towards the first form. The unfolded enzyme, although retaining the functional chemical groups needed for the biological activity, does not maintain them in the appropriate spatial disposition defined as native state. Improperly folded mutant enzymes are usually sensitive to proteolysis and are cleared by the protein quality control systems in the cytosol and endoplasmic reticulum. Activity can be rescued if the equilibrium is pushed back towards the native state. This can be obtained binding a pharmacological chaperone to the folded enzyme. In fact the binding energy of the ligand compensates for the loss in DeltaG of unfolding. Lysosomal alpha-galactosidase represents a good model system for the therapy with pharmacological chaperones. Lysosomal alpha-galactosidase catalyzes the removal of α-galactosyl residues from a glycosphingolipid, globotriaosylceramide. Mutations of lysosomal alpha-galactosidase cause Fabry disease. We use three methods to test the effect of pharmacological chaperones: 1) Thermal shift assay. This test takes advantage of an environmentally sensitive fluorescent dye which binds the enzyme when it reaches the melting temperature; 2) Urea induced unfolding coupled with limited proteolysis and Western blot detection. This test can be carried out on mutants in cell extracts; and 3) Administration of the pharmacological chaperone to cells expressing mutant enzymes. Open reading frames encoding mutated enzymes are introduced into vectors suitable for transient expression. Eukaryotic cells, COS7 or HEK293, are transfected and cultivated in the presence and in the absence of the drug. If the chaperone works and the mutant are stabilized, a larger amount of protein is detected by western blot and consequently a higher enzymatic activity measured.
Zeina Nasr
University of Balamand, Lebanon
Title: Knockdown of RPS3, a DNA repair endonuclease, impedes colon cancer growth and progression by decreasing lactate dehydrogenase activity
Time : 16:00:16:20

Biography:
Zeina Nasr passion is to understand the molecular aspect of tumor initiation and progression. Her research focuses on studying the effect of translation initiation dysregulation on cancer behavior. She has worked with several cell lines and transgenic mouse models and deciphered important pathways that contribute to cancer initiation and progression to metastasis. She built her experience by conducting research and teaching in academic institutions. She is currently interested in understanding the extra-ribosomal functions of ribosomal proteins and their effects on tumorigenesis.
Abstract:
Statement of the Problem: In addition to their role in ribosome biogenesis, ribosomal proteins (RPs) play important roles in DNA repair, proliferation, apoptosis and resistance to drugs and chemotherapy. Ribosomal protein S3 (RPS3), a DNA repair endonuclease, is known to be overexpressed in colon adenocarcinoma. In order to ensure their survival, cancer cells rely on aerobic glycolysis catalyzed by the enzyme lactate dehydrogenase (LDH). Our aim is to identify the role of RPS3 in colon cancer growth and metabolism.
Methodology & Theoretical Orientation: Human colon adenocarcinoma Caco-2 and normal colon NCM-640 cells were tested for the expression of RPS3 by Western blot. In order to inhibit RPS3 expression, cells were transfected with siRNA against RPS3 or a non-targeting siRNA (siNT) as a negative control. Upon RPS3 knockdown, cell behaviors were tested including proliferation and survival by trypan blue and WST-1 assays, and cell migration and invasion by the Boyden chamber assays. The glycolysis state of colon cancer cells was assessed by measuring LDH activity upon RPS3 knockdown using the LDH assay.
Findings: RPS3 was shown to be expressed in both Caco-2 and NCM-640 cells. RPS3 knockdown in Caco-2 significantly reduced cell proliferation, survival, migration and invasion compared to siNT-transfected cells. In NCM-640, RPS3 knockdown did not significantly affect cell proliferation and survival implying that RPS3 expression is selectively crucial for colon cancer cell growth. Interestingly, LDH activity was suppressed upon RPS3 knockdown, suggesting a decrease in glycolysis which explains in part the decrease in proliferation.
Conclusion & Significance: This is the first report that shows a role of RPS3 in regulating LDH activity therefore affecting the glycolytic state, the survival and proliferation of cancer cells. Our results also demonstrate that RPS3 is a selective molecular marker in colon cancer and a potential attractive target for colon cancer therapy.
Biography:
To be Updated...
Abstract:
To be Updated...
Michal Blatkiewicz
Lodz University of Technology, Poland
Title: Continuous methods of fungal laccase concentration
Time : 15:00-15:20

Biography:
Michal Blatkiewicz has done his Master of Engineering Technology in the field of Chemical and Process Engineering from Cracow University. Currently, he is a PhD student at Lodz University of Technology, Faculty of Process and Environmental Engineering, where he is also employed as a Scientific Project Contractor. During his PhD studies, he has done four internships at Dortmund University of Technology. His scientific scope includes primarily fungal cultures and enzymes, and also downstream processing of biological molecules. Currently, he is a part of a research team working on a project concerning continuous processes of biosynthesis, concentration, and purification of fungal laccases, in which he focuses mostly on novel downstream processing methods, such as aqueous two-phase extraction and foam fractionation.
Abstract:
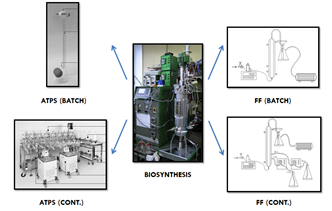
Statement of the Problem: Downstream processing of biological molecules is a very time- and energy-consuming task. One of the major trends in contemporary biotechnology revolves around cost-effective and environment-friendly methods of concentration and purification of bioproducts. Various novel downstream processing tactics are currently being investigated as alternatives to established methods such as ultrafiltration and chromatography. The purpose of this research was to examine the feasibility of polyethylene glycol-phosphate aqueous two phase systems (ATPS) and cetrimonium bromide-induced foam fractionation (FF) as methods for Cerrena unicolor and Pleurotus sapidus laccase separation from culture supernatants. Both processes were investigated in batch and continuous forms.
Methodology: The biosynthesis was performed in a 14-L bioreactor equipped with a set of sensors for process control. The filtered supernatants were concentrated with the use of aqueous two-phase systems or foam fractionation. Batch ATPS experiments were conducted in specially designed extraction flasks, and for continuous ATPS experiments, a mixer-settler unit (MSU) was used. FF experiments were conducted in a special glass column equipped with air disperser and peristaltic pumps for liquid intake and outtake. Laccase activity was determined by 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) assay.
Findings: C. unicolor laccase showed greater affinity towards salt-rich phase with over 90% yields and partitioning coefficients up to 2200. P. sapidus laccase showed strong affinity towards polymer rich-phase also with over 90% yields and full partitioning. MSU experiments showed consistency with batch experiments within non-extreme phase ratio range. Foam fractionation effectiveness depended strongly on pH and surfactant concentration, leading over 100 partitioning coefficient towards the foamate. Low gas and liquid flow rates led to more effective concentration.
Conclusions: Aqueous two-phase extraction and foam fractionation are both effective alternatives to established downstream processing methods for laccase concentration.
- Track 2: Enzymology In Drug Discovery | Clinical Enzymology
Location: Olimpica 1
Session Introduction
Wael M. Rabeh
New York University Abu Dhabi, UAE
Title: The catalytic and structural roles of the Human Hexokinase 2 in cancer

Biography:
Wael Rabeh received his PhD in Biochemistry in the lab of Prof Paul F. Cook where he characterized the last enzymatic reaction of the cysteine biosynthetic pathway in Salmonella typhimurium. In 2005, Dr. Rabeh joined the Structural Genomic Consortium (SGC) at the University of Toronto as a post-doctoral fellow, where he characterized the 3D structure of human proteins with medical relevance using X-ray crystallography. In 2007, Dr. Rabeh joined the lab of Dr. Gergely Lukacs at McGill University for the characterization of a membrane channel that is the main cause of Cystic Fibrosis. Dr. Rabeh’s research is devoted for the characterization of proteins’ structure and mechanism to understand their biological function. Major area of his research focus is devoted for the characterization of disease-causing mechanism of proteins with medical relevance to assist in the discovery and design of new therapeutics using proteins’ structural information and computer simulation.
Abstract:
Glucose metabolism is 200 times higher in cancer in comparison to normal tissue as a strategy to support tumor growth and progression, historically known as the ‘Warburg effect’. Hexokinase is the first enzyme of the glycolytic pathway that catalyzes the phosphorylation of glucose for its activation to glucose-6-phosphate and uses ATP as high-energy source of phosphates. Four isozymes are present in human with Hexokinase 2 (HK2) is most active and specifically expressed in verity of different cancers. However, HK2 binding to the outer mitochondrial membrane not only gives it prime access to ATP generated by the mitochondrial but inhibit apoptosis. Here, we aim to biochemically and structurally characterize interactions of HK2 with the mitochondria and the N-terminal role in catalysis and stability of the full-length enzyme. Here, we solved the crystal structure of human HK2 in complex with glucose and glucose-6-phosphate (PDB code: 2NZT), where it is a homodimer with catalytically active N- and C-terminal domains linked by a seven-turn α-helix. Different from the inactive N-terminal domains of isozymes 1 and 3, the N- domain of HK2 not only capable to catalyze a reaction but it is responsible for thermodynamic stabilizes of full-length enzyme. Deletion of first α-helix of the N-domain that binds to the mitochondria altered the stability and catalytic activity of the full-length HK2. In addition, we found the linker helix between the N- and C-terminal domains to play an important role in controlling the catalytic activity of the N-terminal domain. HK2 is a major step in the regulation of glucose metabolism in cancer making it an ideal target for the development of new anticancer therapeutics. Characterizing the structural and molecular mechanisms of human HK2 and its role in cancer metabolism will accelerate the design and development of new cancer therapeutics that are safe and cancer specific.
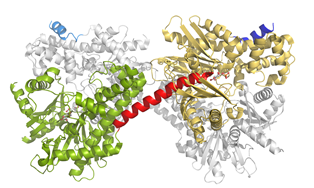
References:
- Rabeh, W.M.; Bossard, F.; Xu, H.; Okiyoneda, T.; Bagdany, M.; Liu, Y.; Mulvihill, C.M.; Du, K.; Di Bernardo, S.; Konermann, L.; Roldan, A.; Lukacs, G.L. Correction of both NBD1 energetics and domain interface is required to restore ∆F508 CFTR folding and function. (2012) Cell; 148(1-2): 150-63.
- Wyatt, E.; Wu, R.; Rabeh, W.; Park, H.W.; Ghanefar, M. and Ardehali, H. Regulation and Cytoprotective Role of Hexokinase III. (2010) PLoS One. 5(11): e13823
- Okiyoneda, T.; Barrière, H.; Bagdány, M.; Rabeh, W.M.; Du, K.; Höhfeld, J.; Young, J.C. and Lukacs, G.L. Peripheral Protein Quality Control Removes Unfolded CFTR from the Plasma Membrane. (2010) Science. 329: 805-10.
- Rabeh, W.M.*, Tempel, W.*, Bogan, K.L., Belenky, P., Wojcik, M., Seidle, H.F., Nedyalkova, L., Yang, T., Sauve,A.A., Park, H.W. and Brenner,C. Nicotinamide Riboside Kinase Structures Reveal New Pathways to NAD+. (2007) PLoS Biol. 5(10): e263 *Co-first Authors.
- Rabeh, W.M.*, Chattopadhyay, A.*, Meier, M., Ivaninskii, S., Burkhard , P., Speroni, F.*, Campanini, B., Bettati, S., Mozzarelli, A., Li, L. and Cook, P.F. Structure, Mechanism, and Conformational Dynamics of O-Acetylserine Sulfhydrylase from Salmonella typhimurium: Comparison of A and B Isozymes. (2007) Biochemistry 46, 8315-30 *Co-first Authors.
Anna Antecka
Lodz University of Technology, Poland
Title: Upstream and downstream processing of fungal laccase

Biography:
Anna Antecka has received her PhD in Environmental Engineering in 2008 from the Lodz University of Technology in Poland, within the Faculty of Process and Environmental Engineering, where she is currently an Assistant Professor in the Department of Bioprocess Engineering. From 2004-2005, she made a one-year scientific stay at the International Institute Zittau, Germany, in the Department of Environmental Biotechnology. In 2002, she studied at the University of Dortmund, Germany. Her main research interests are in microbial ecology and biotechnology of fungal enzymes especially laccase, including its production, purification and characterization as well as enzyme applications for industrial and environmental purposes. Currently, she is focused on projects in the area of integrated continuous up- and downstream processes for the biosynthesis and purification of fungal laccases.a
Abstract:
Statement of the Problem: Laccase (EC 1.10.3.2, polyphenol oxidases) belongs to the group of oxidoreductases which is characterized by its specific catalytic properties and the ability to oxidize various organic compounds. Therefore the enzyme is very attractive for a wide range of industrial and environmental purposes. However, due to relatively low effectiveness and the possibility of gradual degradation of bioproducts in the reactor or during the separation and purification stages, there is a need for new approaches and research in this field. Therefore, the purpose of this research was to study and integrate the stages of up- and downstream processing (biosynthesis and purification) of laccase from Cerrena unicolor in order to obtain a highly active enzymatic product.
Methodology: The biosynthesis was performed in a 14-L bioreactor equipped with a set of sensors for process control. Modifications to the medium- addition of microparticles, MPEC, as well as various types of cultivation/growth strategies were examined. The supernatant was concentrated and purified by an aqueous two-phase system (ATPS) consisting of polyethylene glycol and phosphate buffer solutions, and through foam fractionation (FF) at different pH values and with the addition of different detergents. Ultrafiltration and chromatography methods were also investigated. Molecular mass and isoelectric point was determined with the use of electrophoresis.
Findings: Laccase activity increased 3.5-fold after addition of microparticles to the culture media. The fed-batch mode resulted in high laccase activity (up to 4 U/mL) which remains stable during cultivation. The optimal conditions for laccase purification by FF and ATPS were determined with activity partitioning coefficients between foamate and retentate of almost 200 and 2000, respectively, and with yields reaching 50% and 90%, respectively.
Conclusions: Application of MPEC and fed-batch mode proved successful in increasing enzyme production. Both ATPS and FF are successfully applicable for laccase purification.
References:
1. Antecka A, Blatkiewicz M, Bizukojc M, Ledakowicz S (2016) Morphology engineering of basidiomycetes for improved laccase biosynthesis. Biotechnol. Lett. 38:667-672.
2. Antecka A, Bizukojć M, Ledakowicz S (2009) The kinetic model of laccase biosynthesis by Cerrena unicolor. Chemical, Process Engineering 30:403-416.
3. Michniewicz A, Ledakowicz S, Ullrich R, Hofrichter M (2008) Kinetics of the enzymatic decolorization of textile dyes by laccase from Cerrena unicolor. Dyes and Pigments 77:295-302.
4. Sójka-Ledakowicz J, Lichawska-Olczyk J, Ledakowicz S, Michniewicz A (2007) Bio-scouring of linen fabrics with laccase complex from Cerrena unicolor. Fibres and Textiles EE 63:86-89.
5. Michniewicz A, Ullrich R, Ledakowicz S, Hofrichter M (2006) The white-rot fungus Cerrena unicolor strain 137 produces two laccase isoforms with different physico-chemical and catalytic properties. Applied Microbiology and Biotechnology 69:682-688.
Michal Blatkiewicz
Lodz University of Technology, Poland
Title: Continuous methods of fungal laccase concentration

Biography:
Michal Blatkiewicz has done his Master of Engineering Technology in the field of Chemical and Process Engineering from Cracow University. Currently, he is a PhD student at Lodz University of Technology, Faculty of Process and Environmental Engineering, where he is also employed as a Scientific Project Contractor. During his PhD studies, he has done four internships at Dortmund University of Technology. His scientific scope includes primarily fungal cultures and enzymes, and also downstream processing of biological molecules. Currently, he is a part of a research team working on a project concerning continuous processes of biosynthesis, concentration, and purification of fungal laccases, in which he focuses mostly on novel downstream processing methods, such as aqueous two-phase extraction and foam fractionation.
Abstract:
Statement of the Problem: Downstream processing of biological molecules is a very time- and energy-consuming task. One of the major trends in contemporary biotechnology revolves around cost-effective and environment-friendly methods of concentration and purification of bioproducts. Various novel downstream processing tactics are currently being investigated as alternatives to established methods such as ultrafiltration and chromatography. The purpose of this research was to examine the feasibility of polyethylene glycol-phosphate aqueous two phase systems (ATPS) and cetrimonium bromide-induced foam fractionation (FF) as methods for Cerrena unicolor and Pleurotus sapidus laccase separation from culture supernatants. Both processes were investigated in batch and continuous forms.
Methodology: The biosynthesis was performed in a 14-L bioreactor equipped with a set of sensors for process control. The filtered supernatants were concentrated with the use of aqueous two-phase systems or foam fractionation. Batch ATPS experiments were conducted in specially designed extraction flasks, and for continuous ATPS experiments, a mixer-settler unit (MSU) was used. FF experiments were conducted in a special glass column equipped with air disperser and peristaltic pumps for liquid intake and outtake. Laccase activity was determined by 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) assay.
Findings: C. unicolor laccase showed greater affinity towards salt-rich phase with over 90% yields and partitioning coefficients up to 2200. P. sapidus laccase showed strong affinity towards polymer rich-phase also with over 90% yields and full partitioning. MSU experiments showed consistency with batch experiments within non-extreme phase ratio range. Foam fractionation effectiveness depended strongly on pH and surfactant concentration, leading over 100 partitioning coefficient towards the foamate. Low gas and liquid flow rates led to more effective concentration.
Conclusions: Aqueous two-phase extraction and foam fractionation are both effective alternatives to established downstream processing methods for laccase concentration.
Jennifer A Littlechild
University of Exeter, UK
Title: Thermophilic enzymes as industrial biocatalysts

Biography:
http://enzymology.conferenceseries.com/Jennifer A Littlechild is Professor of Biological Chemistry and has established the Henry Wellcome Centre for Biocatalysis at Exeter University in 2003. Her research studies involve the structural and mechanistic characterisation of a range of enzymes from thermophilic bacteria and Archaea that have industrial applications. She has a particular interest in thermophilic carbonic anhydrase enzymes and has carried out a project with Statoil from 2011-2013. She has published over 200 publications in refereed high impact journals and presented her research work internationally. She is the UK Representative and Vice-chair of the European Section of Applied Biocatalysis and Member of EU advisory committees for industrial biotechnology.
Abstract:
There is an increasing demand for new enzymes with enhanced performance and/or novel functionalities that provide savings in time, money and energy for industrial processes in the areas of high value chemical production and other "white" biotechnology applications. There is a limited understanding of the metabolic capacity of life and only a small proportion of nature’s catalysts have been utilised for industrial biotechnology. There are new metabolic pathways and enzyme activities to be discovered and many of which could be identified within the large proportion of micro-organisms that cannot be cultured and within their associated viruses. The number of enzymes explored to date remains within the range of 1-2% of known microbial diversity. Enzymes used for commercial biotransformation reactions are required to be stable under the industrial conditions employed. The use of naturally thermostable enzymes isolated from hot environments can be a source of enzymes that are more stable to high temperatures, extremes of pH and exposure to organic solvents. By using both genomic and metagenomic approach within the projects, HOTZYME and THERMOGENE, we have identified hydrolase and transferase enzymes of industrial interest isolated from high temperature environments around the world. A selection of these novel enzymes including esterases, cellulases, epoxide hydrolases, transketolases and transaminases have been characterized both biochemically and structurally. In the case of the epoxide hydrolases, two new enzymes with interesting substrate specificity and stereo-selectivity have been discovered from thermophilic metagenomes. Applications of these new epoxide hydrolases have been demonstrated at industrial scale for the production of new chiral chemical building blocks. A new thermophilic cellulase enzyme with activity at pH 5.0 and active under high salt conditions has been isolated which has potential applications for breakdown of biomass.
References:
- Sayer C, Finnigan W, Isupov M, Levisson M, Kengen S, Van der Oost J, Harmer N, Littlechild J A (2016) Structural and biochemical characterization of Archaeoglobus fulgidus esterase reveals a bound CoA molecule in the vicinity of the active site. Scientific Reports 6:25542.
- Zarafeta D, Kissas D, Sayer C, Gudbergsdottir S R, Ladoukakis E, Isupov M N, Chatziioannou A, Peng X, Littlechild J A, Skretas, G, Kolisis F N (2016) Discovery and characterization of a thermostable and highly halotolerant GH5 Cellulose from an Icelandic Hot Spring Isolate. PLoS ONE 11(1):07.
- Sayer C, Szabo Z, Isupov M N, Ingham C, Littlechild J A (2015). The structure of a novel thermophilic esterase from the Planctomycetes species, Thermogutta terrifontis reveals an open active site due to a minimal cap domain. Front. Microbiol. 11: 1294.
- Ferrandi C, Sayer C, Isupov M, Annovazzi, C, Marchesi C, Lacobone G, Peng X, Bonch Osmolovskaya E, Wohlgemuth R, Littlechild J, Monti D (2015) Discovery and characterization of thermophilic limonene-1,2-epoxide hydrolases from hot spring metagenomics libraries. FEBS J. 282:2879-2894.
- Sayer C, Isupov M, Bonch-Osmolovskaya E, Littlechild J A ( 2015) Structural studies of a thermophilic esterase from a new Planctomycetes species, Thermogutta terrifontis. FEBS J. 282: 2846-2857
Preeti Chanalia
Kurukshetra University, India
Title: Proline iminopeptidase from probiotic L. Plantarum: Physicochemical characterization and role in meat tenderization
Time : 11:40-12:00

Biography:
Preeti Chanalia has acquainted herself with biochemical, microbiological and microscopic techniques. She is well versed with protein isolation and purification. She has purified and characterized PIP from L. plantarum and extended her studies to explore applications of PIP (alone and in combination with other enzymes) in meat tenderization and collagen degradation. Her work determined that purified PIP as well membrane bound PIP was very effective in collgen degradation and meat tenderization. Her work indicates the possibility of formation of monostrains into multi-strain probiotics with improved efficiency. She had made a significant contribution to the project and worked as self-motivated researcher to learn and do new things. This study is very useful for food and pharmaceutical industry.
Abstract:
Proline iminopeptidase (PIPs) specifically cleaves N-terminal proline from peptides and helps in overcoming restriction of many aminopeptidases to cleave proline rich peptides/proteins. PIPs are used in food processing, meat tenderization as well as in treating collagen rich wastewater to generate bioactive collagen hydrolysates important for food and pharmaceutical industry. Microbial enzymes account for about 60% of total worldwide sale of enzymes. There is increasing demand of industrial enzymes with novel characteristics for different applications. Among microorganisms lactic acid bacteria have gained great attention because of their GRAS status (generally regarded as safe) and probiotic attributes. Investigation and development of highly potent probiotic consortium (comparable to commercially available products/Probiotics) suitable for functioning in adverse conditions is of great value as therapeutics as well as in various industries. The possibility of formation of mono- strains into multi-strain probiotics with potential of improved efficacy is the goal of this kind of studies. Methodology: Purification using successive column chromatographies, characterization, in silico studies of PIP from probiotic L. plantarum and its use in collagen degradation and meat tenderization by simple and effective treatment with enzyme/s was done. Findings: Membrane bound PIP from L. Plantarum was extracted, purified and characterized further. The effectiveness of PIP in purified form, membrane bound form and in combination with other enzymes to degrade collagen and meat tenderization marks its industrial importance. Conclusion & significance: Purified PIP as well as whole cells effectively hydrolyzed collagen. Whole cells, PIP alone and with other enzymes tenderized chicken meat efficiently. It is very significant for food, pharmaceutical industry and waste management. It also opens avenue for the formation of multi strain probiotics.
References:
- Dimpi Gandhi, Preeti Chanalia, Pooja Attri, Suman Dhanda. Dipeptidyl peptidase-II from probiotic Pediococcus acidilactici: Purification and functional characterization. International Journal of Biological Macromolecules. 2016, 93,919:932.
- Preeti Chanalia, Dimpi Gandhi, Raman Kumar, Pooja Attri, Suman Dhanda. Lactic acid production vis-à-vis biowaste management using lactic acid bacteria. World Applied Science Journal. 2016.(Accepted)
- Pooja Attri, Drukshakshi Jodha, Dimpi Gandhi, Preeti Chanalia, Suman Dhanda. In vitro evaluation of Pediococcus acidilactici NCDC 252 for its probiotic attributes. International Journal of Dairy Technology. February 2015. DOI: 10.1111/1471-0307.12194.
- Prabodh Chander Sharma, Ankit Jain, Mohammad Shahar Yar, Rakesh Pahwa, Jasbir Singh, Preeti Chanalia. Novel fluoroquinolone derivatives bearing N-thiomide linkage with 6-substituted-2-aminobenzothiazoles: synthesis and antibacterial evaluation. Arabian Journal of Chemistry. November 2012. DOI: 10.1016/j.arabjc.2012.11.002.
- Preeti Chanalia, Dimpi Gandhi, Drukshakshi Jodha, Jasbir Singh. Applications of microbial proteases in pharmaceutical industry: an overview. Reviews in medical microbiology. 2011, 22: 96-101.
Dino Hasanagic
University of Banja Luka, Bosnia and Herzegovina
Title: The role of Zeolite in reducing oxidative damage in tomato plants exposed to drought
Time : 12:00-12:20

Biography:
Dino Hasanagic received his degree of M.Sc. from the Faculty of Science in Sarajevo, and he is currently attending plant biochemistry programme at Faculty of Science in Novi Sad, Serbia. His field of interest is antioxidative metabolism of plant cell - effects of abiotic stress (drought, metals, senescence, ROS signaling, redox state). Dr Biljana Kukavica is expert in plant enzyme antioxidative metabolism. Her interest is focused on enzyme and non-enzyme antioxidative defense in plant cell under oxidative stress. Dr Danijela Kojic has a big experience in the field of biochemistry and molecular biology and her researches are addressing the antioxidative enzyme role in adaptation to stress conditions. The expertise and passion of the authors is recognized among their peers, and they constantly expanding their knowledge in researches regarding enzymology.
Abstract:
The drought is a worldwide problem and insufficient supply of plants with water is one of the most important causes of low agricultural yields and thus representing one of the most common problems faced by the producers. There has been an increased interest in science in recent years in the use of natural aluminosilicates in agriculture where the most famous is zeolite, a mineral whose absorption properties and balanced release of water and nutritive substances ever more successfully solve the issue of water supplying and mineral nutrition and have beneficial impact on overall plant growth. The aim of this study was to investigate the role of zeolite in prevention of oxidative stress in tomato plants exposed to drought. Changes in the activity of peroxidase (POD, EC 1.11.1.7), catalase (CAT, EC 1.11.1.6), ascorbate peroxidase (APX, EC 1.11.1.11), superoxide dismutase (SOD, EC 1.15.1.1) as well as reduced and total ascorbate content in plant leaves exposed to drought for 28 days were investigated. Activities of antioxidant enzymes in the leaves of plants exposed to drought were at the same level with and without the addition of zeolite. The obtained results indicate that zeolite did not prevent oxidative damages caused by drought. Native electrophoresis resolved the presence of two peroxidase isoforms specific for drought and their activities were higher in tomato leaves whit zeolite. The drought induced an increase in activities of superoxide dismutase, ascorbate peroxidase and ascorbate concentration and this antioxidative strategy was more expressed in zeolite treated plants. Unexpected results related to the role of zeolite open the possibility to different perspectives in discussion on the zeolite role in drought prevention.
References:
- Alfi S, Azizi, F (2015) Effect of Drought Stress and Using Zeolite on Some Quantitative and Qualitative Traits of Three Maize Varieties. Research Journal of Recent Sciences 4: 1-7.
- Chavan, ML, Janagoudar, BS, Dharmatti, PR, Koti, RV (2010) Effect of drought on growth attributes of tomato (Lycopersicon esculentum Mill.) genotypes. Indian Journal of Plant Physiology 15: 11-18.
- Kojić, D, Pajević, S, Jovanović-Galović, A, Purać, J, Pamer, E, Škondrić, S, Milovac, S, Popović, Ž, Grubor-Lajšić, G (2012) Efficacy of natural aluminosilicates in moderating drought effects on the morphological and physiological parameters of maize plants (Zea mays L.). Journal of Soil Science and Plant Nutrition 12: 113-123.
- Pirzad, A, Mohammadzade, S (2014) The effects of drought stress and zeolites on the protein and mineral nutrients of Lathyrus sativus. International Journal of Biosciences 4: 241-248.
- Gholizadeh A, Amin, MSM, Anuar, AR, Saberioon, MM. (2010) Water stress and natural zeolite impacts on physiomorphological characteristics of Moldavian Balm (Dracocephalum moldavica L.). Australian Journal of Basic and Applied Sciences 4: 5184- 5190
Muhammad Nauman Aftab
Government College University, Pakistan
Title: Cloning, expression and characterization of β-xylanase gene from Thermotoga naphthophila
Time : 14:20-14:40
Biography:
Dr. Muhammad Nauman Aftab is an associate professor at MN Institute of Industrial Biotechnology, Government College University, Lahore, Pakistan. He has worked in the area of cloning and gene expression of Bacillus licheniformis and Geobacillus stearothermophilus that code for industrially important enzymes. Some of the cloned genes by his team are β-glucanase, serine protease, β-glucosidase, xylanase, etc. Apart from cloning genes, he has also worked on the production of various enzymes like tannase, lipase and polypeptide antibiotic (bacitracin) from wild bacterial strains like Bacillus licheniformis, Bacillus subtilis and Geobacillus stearothermophilus.
Abstract:
More than 90% energy requirements depend on fossil fuels that include crude oil, natural gas, coal, etc. The burning of these energy sources emit greenhouse gases that are harmful for our environment and are major cause of many diseases like lung cancer, asthma etc. The requirement of fossil fuel is increasing day by day due to rapid industrialization and increased number of vehicles. In the middle of this century, world’s fossil fuels reserves will be significantly reduced. In this scenario, the alternative fuel, bioethanol, can serve as an ideal candidate in reducing greenhouse emissions and can also help in fulfilling the partial energy needs globally. To achieve bioethanol, highly thermostable β-xylanase gene Thermotoga naphthophila was cloned and expressed in E. coli BL21 utilizing pET-21a(+) expression vector. To obtain maximum expression of recombinant xylanase, growth conditions i.e., temperature, pH, inducer and induction time of E. coli were optimized and found to be 7.0, 37ºC, 0.5 mM and 4 hours, respectively. Heat treatment was used for the partial purification of recombinant enzyme. Further purification was carried out by ammonium sulphate precipitation followed by the anion exchange chromatography. Molecular weight of pure recombinant β-xylanase enzyme was calculated to be 150 kDa by SDS-PAGE. Considerable stability was exhibited by the purified enzyme at pH value 8.0 and at temperature 90ºC. The activity of the enzyme was decreased considerably in the presence of EDTA and significantly increased in the presence of Cu+2. The effect of different organic solvents (methanol, ethanol, n-butanol, acetone and isopropanol) was also explored but no considerable effect on activity of xylanase enzyme was observed. Similarly, inhibitors (urea, triton X-100, Tween-20, Tween-80 and β-mercaptoehanol) did not have any considerable effect on the enzyme activity, however, addition of SDS significantly reduced the activity of β-xylanase activity. The β-xylanase enzyme obtained can be utilized for the saccharification of lignocellulosic mass which can in turn be used in the production of bioethanol.
Aida M Farag
Alexandria University, Egypt
Title: Biobanking of stem cells: Purification, characterization and application of marine Aspergillus nomius GWA5 tannase
Time : 16:10-16:30

Biography:
Aida Farag has her expertise in production of important enzymes used in treatment as tannase. We used a marine environment as a new source for isolation of a fungus able to produce the enzyme. Also, the fungus was identified as Aspergillus nomius GWA5. Partial purification by different agents was done followed by purification using two types of columns and characterization of the enzyme was also studied. The properties of Aspergillus nomius GWA5 tannase may make it very good candidate for using in biotechnology field.
Abstract:
Tannase ) EC 3.1.1.20) was produced from the culture filtrate of marine Aspergillus nomius GWA5 and purified by 75% acetone precipitation, followed by gel filtration on Sephadex G-100 and on DEAE-Sephadex A-50 ion exchange chromatography, yielding 4.48-fold of purification. The molecular weight of the purified tannase was 30kDa, determined by a sodium dodecyl sulphate polyacrylamide gel electrophoresis. The optimum pH and temperature of the purified tannase yielding the highest activity (291 U/mg protein) were 6.0 and 50ºC, respectively. Tannase stability shifted to a more acidic range (4-6). Tannase enzyme was fairly stable to heat treatment in absence of its substrate and it retained about 84.5% of its activity when treated at 80 ºC for 15 min. The effect of activators and inhibitors on tannase activity was investigated, only Mg2+activated the pure enzyme while Cd2+, EDTA, Pb2+ and Hg2+ inhibited the enzyme activity and it retained about 51.55%, 40.78%, 30.24% and 24.55% of its activity, respectively. Tannase showed promising activity in removing tannin stains of tea from clothes.
Hala Mourad Demerdash
Alexandria University Hospitals, Egypt
Title: Enzymatic changes in obesity-related diseases
Time : 16:30-16:50
Biography:
Hala Mourad Demerdash is currently an Associate Professor in the Faculty of Pharmacy, Pharos University in Alexandria (PUA) and Consultant in Clinical Pathology at Alexandria University Hospital. She graduated from the Faculty of Medicine Alexandria University and has received her Master’s degree from Chemical Pathology department, Medical Research Institute and she was a Resident in the Chemical Pathology department from1992-1995. She received her MD from Clinical Pathology Department, Faculty of Medicine, Tanta University in 2007. She was an Associate Professor in 2014 at Tanta University.
Abstract:
Obesity is a condition of abnormally increased body fat, resulting from increased energy intake relative to energy expenditure. Obesity is defined as having a body mass index (BMI) of 30 kg/m2 or above, with an increasing incidence in all over the world. Obesity is associated with several health related problems that increase the risk of morbidity and mortality. Scientists have found that enzymes play an important role in obesity-related diseases. They found that at very low, basal levels, enzymes control the threshold of cellular function “signaling pathways”. The purpose of this review is to describe the role of oxidative stress in various obesity related health problems; since obesity is associated with oxidative stress as result of increased oxygen utilization due fat deposition in tissues, increased cardiac load, coupled with oxidative phosphorylation in mitochondria and depletion of ATP and consequent free radical formation. Moreover, high dietary saturated fatty acids (SFA) stimulate intracellular pathways, with more oxidative stress through superoxide generation from NADPH oxidases, glyceraldehyde autoxidation, protein kinase C (PKC) activation, and polyol pathway. In addition, oxidative stress is associated with the infiltration of adipose tissue by inflammatory cells, together with production of high levels of free radical as part of the immune response. Thus obesity is viewed as a chronic inflammatory situation that is critically important in development of pathological states due to tissue damage and altered enzyme activity that result in disease state to extent of tumorigenesis, such as insulin resistance diabetes, cardiovascular disease and kidney dysfunction. It can be concluded that weight reduction in obese subjects improve anti-oxidant defense of body; the triggering factor for series of molecular signals. Also, improving anti-oxidant defense through modulation of dietary pattern or pharmaceutical antioxidants, which may be a potential therapeutic approach in obesity related disease.
Ebru Sakar
Harran University, Turkey
Title: Selection based breeding and genetic characterization of selected olive (Olea Europaea L.) genotypes from Adiyaman, Mardin, Siirt, Åžanliurfa and Åžirnak provinces
Time : 11:20-11:40

Biography:
Ebru Sakar is associate professor at Harran University Faculty of Agriculture, in the Department of Horticulture Osmanbey Campus, Turkey. He did his masters in gardens plants from Harran University, Turkey. His research interest is Fruit Growing and Breeding Biotechnology.
Abstract:
Present study was aimed to select superior genotypes within olive populations of Adıyaman, Mardin, Åžanlıurfa and Şırnak provinces in South East Anatolia, leading fruit and tree characteristics were determined in shoot, leaf and fruit samples collected from 142 genotypes. These genotypes were investigated according to “Weighted Rankit” method using fruit weight, number of fruits per 100g, flesh/seed ratio, oil ratio, fatty acid composition, habitus, lenticel size, length of internode and 38 promising genotypes as cultivar candidate (20 are table olives and 18 are olive oil) were selected. Among table genotypes, number of fruits per 100 g between 11 (Yurteri-4) and 25 (Derik-20 and Yedi kardeÅŸler-1), flesh/seed ratio between 5.85g (Yardere-2) and 11.40g (Yedi kardeÅŸler-3) and oil content between 3.0% (Eski kale) and 13.0% (Amak-1); among oil genotypes, fruit weight between 1.14g (Derik zeytin pınarı-2) and 8.99 g (Yurteri-4), flesh/seed ratio between 2.73 g (Zinnar-5) and 11.40 g (Yedi kardeÅŸler-3); oil content between 2.0% (Eski kale çıkışı) and 13.0% (Amak-1), oleic acid ratio between 63.25% (GürmeÅŸe-2) and 74.31% (Yurteri-6) and linolenic acid ratio between 0.49% (Yedi kardeÅŸler-1,Eski kale çıkışı) and 1.42% (BeÅŸdeÄŸirmen-2) were changed. Genetic characterization of 38 selected table and olive (Olea europaea L.) genotypes together with 6 local and 4 foreign reference cultivars obtained from Alata Horticultural Institute was performed using 10 microsatellite markers ((UDO4, UDO9, UDO12, UDO24, UDO26, DCA9, DCA11, DCA13, DCA15 and DCA18) and their genetic similarities were investigated by SSR method. Number of alleles per locus was determined between 7 (UDO9 and UDO24) and 16 (DCA18) for table genotypes and between 4 (UDO4) and 15 for oil genotypes (DCA11ve DCA18). Mean expected and observed heterozygosity were determined as 0.694-0.604 among table genotypes and 0.710 and 0.656 among oil genotypes, respectively. The dendrogram obtained from cluster analysis consisted of two main groups. Several sub-groups were observed within the first group which consisted of the majority of genotypes. This data was further supported by the AFLP analysis. Homonyms and synonyms were not obtained. Close genetic similarities were determined between Amak-1 and Yedi kardeÅŸler-3 in table genotypes and between. Zinnar-5 and Yurteri-4, between. GürmeÅŸe-1 and GürmeÅŸe-2 in oil genotypes.
- Special Session
Location: Olimpica 1
Session Introduction
Zeina Nasr
University of Balamand, Lebanon
Title: The extraribosomal role of ribosomal proteins
Time : 14:40-15:40

Biography:
Zeina Nasr passion is to understand the molecular aspect of tumor initiation and progression. Her research focuses on studying the effect of translation initiation dysregulation on cancer behavior. She had worked with several cell lines and transgenic mouse models and deciphered important pathways that contribute to cancer initiation and progression to metastasis. She built her experience by conducting research and teaching in academic institutions. She is currently mainly interested in understanding the extraribosomal functions of ribosomal proteins and their effects on tumorigenesis.
Abstract:
Protein synthesis is a highly regulated and coordinated process involving the action of ribosomes and a set of translation factors. Ribosome biogenesis occurs in the nucleolus and requires the action of 80 ribosomal proteins (RPs), 4 ribosomal RNAs (rRNAs), other associated proteins and small nucleolar RNAs. The structure of the ribosomal subunits has identified the role of RPs as RNA chaperones for ribosomal assembly, and as endo- and exonucleases essential for the maturation of rRNAs. Some play a role in the joining of 40S and 60S subunits during translation initiation. Others interact with tRNA or stabilize the ribosome by encasing the exit groove. Studies have also shown that RPs may have extraribosomal functions, ranging from DNA repair to replication, proliferation, apoptosis, and chemoresistance. Mutations in RPs in animal models and humans induced a wide variety of phenotypes suggesting a role of RPs beyond the ribosome structure. Thus far, 11 RP mutant mice have been reported exhibiting diverse phenotypes including decrease body size, defective organs, and embryonic lethality. Defects in ribosome biogenesis have been linked to many diseases collectively named ribosomopathies. These include Myelodysplatic Syndromes, due to RPS14 haploinsufficiency and Diamond Blackfan Anemia, caused by mutations in RPS19 gene. These abnormalities have shown an increase susceptibility to hematological malignancies. Indeed, RPs have been linked to tumorigenesis in several reports, suggesting a role in promoting transformation. Several RPs are overexpressed upon activation of the oncogene Myc. Some are found overexpressed in various human tumors, including prostate and colon cancer, metastatic nasopharyngeal carcinomas, metastatic melanomas and metastatic human breast cancer cells. Some RPs have also been proposed as biomarkers for various cancers, such as colorectal, gastric, prostate cancers and lymph node metastasis. These pieces of evidence suggest that RPs could be used as potential targets in cancer therapeutics.
- Young Research Forum
Location: Olimpica 1
Session Introduction
Dino Hasanagic
University of Banja Luka, Bosnia and Herzegovina
Title: The role of Zeolite in reducing oxidative damage in tomato plants exposed to drought

Biography:
Dino Hasanagic received his degree of M.Sc. from the Faculty of Science in Sarajevo, and he is currently attending plant biochemistry programme at Faculty of Science in Novi Sad, Serbia. His field of interest is antioxidative metabolism of plant cell - effects of abiotic stress (drought, metals, senescence, ROS signaling, redox state). Dr Biljana Kukavica is expert in plant enzyme antioxidative metabolism. Her interest is focused on enzyme and non-enzyme antioxidative defense in plant cell under oxidative stress. Dr Danijela Kojic has a big experience in the field of biochemistry and molecular biology and her researches are addressing the antioxidative enzyme role in adaptation to stress conditions. The expertise and passion of the authors is recognized among their peers, and they constantly expanding their knowledge in researches regarding enzymology.
Abstract:
The drought is a worldwide problem and insufficient supply of plants with water is one of the most important causes of low agricultural yields and thus representing one of the most common problems faced by the producers. There has been an increased interest in science in recent years in the use of natural aluminosilicates in agriculture where the most famous is zeolite, a mineral whose absorption properties and balanced release of water and nutritive substances ever more successfully solve the issue of water supplying and mineral nutrition and have beneficial impact on overall plant growth. The aim of this study was to investigate the role of zeolite in prevention of oxidative stress in tomato plants exposed to drought. Changes in the activity of peroxidase (POD, EC 1.11.1.7), catalase (CAT, EC 1.11.1.6), ascorbate peroxidase (APX, EC 1.11.1.11), superoxide dismutase (SOD, EC 1.15.1.1) as well as reduced and total ascorbate content in plant leaves exposed to drought for 28 days were investigated. Activities of antioxidant enzymes in the leaves of plants exposed to drought were at the same level with and without the addition of zeolite. The obtained results indicate that zeolite did not prevent oxidative damages caused by drought. Native electrophoresis resolved the presence of two peroxidase isoforms specific for drought and their activities were higher in tomato leaves whit zeolite. The drought induced an increase in activities of superoxide dismutase, ascorbate peroxidase and ascorbate concentration and this antioxidative strategy was more expressed in zeolite treated plants. Unexpected results related to the role of zeolite open the possibility to different perspectives in discussion on the zeolite role in drought prevention.


Biography:
Francesca Mangiavacchi has received her Master’s degree in 2016 under the supervision of Professor Antimo Gioiello and Claudio Santi in the Department of Pharmaceutical Sciences of University of Perugia. Currently, she is a Research Fellow in the group of Catalysis and Organic Green Chemistry of Professor Claudio Santi working on the development of new catalytic methods for oxidation of natural derivatives under mild conditions. In 2015, she received the award for the Best Oral Communication at the 4th Workshop on Selenium Sulphur and Redox Catalysis and in 2016 a Travel Fellowship from the University of Melbourne to attend at the International Conference on Selenium and Tellurium in Gifu (Japan) under the project Selenium (Redox) Therapeutics.
Abstract:
Oxidation reactions are a central and general field of organic and industrial chemistry, being defined as: “a process which involves a gain in oxygen and/or loss of hydrogen”. In recent years, a wide range of oxidative procedure has been developed in the attempt of synthetic chemist to satisfy the need of reaction efficiency and with regard of environmental impact. Although a great effort has been made, the development of new synthetic green processes is desirable. In pursuing our interest in green chemistry combined with our expertise in the selenium chemistry we have developed new eco-friendly oxidative process taking inspiration by biological mechanism of glutathione peroxidase (GPx). The catalytic cycle of GPx is an oxidation reaction in which the selenium atom of a selenocysteine is the catalyst; the hydrogen peroxide is the stoichiometric oxidant, and glutathione the substrate that is subjected to oxidation. In the native enzyme the catalytic triad prevents over oxidation of selenium that is able to oxidize only the thiols. In the absence of thiols and in the absence of stabilizing interaction small selenium containing compound are oxidized to selenonic or perseleninic acid and in this form they can transfer oxygen to carbon-carbon double bond and carbon oxygen double bond of aldehydes. Interestingly, these reactions can be carried out in “on-water” conditions having as unique side product a molecule of water. Herein we present some example of “bio-logical” oxidation applied to unsaturated organic substrates for the synthesis of diol, oxidation of aldehydes into carboxylic acids and oxidative cyclofunctionalization. We used catalytic amount of selenium based catalyst, as selenocysteine and diphenyl diselenide, and hydrogen peroxide as stoichiometric co-oxidant as greener oxidant. Furthermore, we use mild condition, and we could recycle the catalyst at least five times without an evident loss in terms of yields.