Biography
Biography: Francesca Mangiavacchi
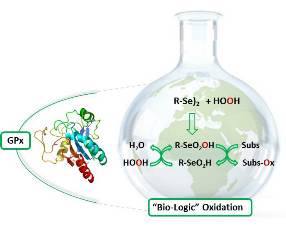
Abstract
Oxidation reactions are a central and general field of organic and industrial chemistry, being defined as: “a process which involves a gain in oxygen and/or loss of hydrogen”. In recent years, a wide range of oxidative procedure has been developed in the attempt of synthetic chemist to satisfy the need of reaction efficiency and with regard of environmental impact. Although a great effort has been made, the development of new synthetic green processes is desirable. In pursuing our interest in green chemistry combined with our expertise in the selenium chemistry we have developed new eco-friendly oxidative process taking inspiration by biological mechanism of glutathione peroxidase (GPx). The catalytic cycle of GPx is an oxidation reaction in which the selenium atom of a selenocysteine is the catalyst; the hydrogen peroxide is the stoichiometric oxidant, and glutathione the substrate that is subjected to oxidation. In the native enzyme the catalytic triad prevents over oxidation of selenium that is able to oxidize only the thiols. In the absence of thiols and in the absence of stabilizing interaction small selenium containing compound are oxidized to selenonic or perseleninic acid and in this form they can transfer oxygen to carbon-carbon double bond and carbon oxygen double bond of aldehydes. Interestingly, these reactions can be carried out in “on-water” conditions having as unique side product a molecule of water. Herein we present some example of “bio-logical” oxidation applied to unsaturated organic substrates for the synthesis of diol, oxidation of aldehydes into carboxylic acids and oxidative cyclofunctionalization. We used catalytic amount of selenium based catalyst, as selenocysteine and diphenyl diselenide, and hydrogen peroxide as stoichiometric co-oxidant as greener oxidant. Furthermore, we use mild condition, and we could recycle the catalyst at least five times without an evident loss in terms of yields.