
Rachel Chen
Georgia Institute of Technology, USA
Title: Metabolic engineering strategies for effective use of glycosyltransferases in oligosaccharide
Biography
Biography: Rachel Chen
Abstract
As one of the four building blocks of life, sugar molecules permeate almost all aspects of life. The widespread occurrence of glycosylation and its broad impact in biological processes underscores the importance of studying glycosylation. To study glycans and probe their roles in a biological system significant amount of pure molecules are needed. Besides basic research, there are a wide range of opportunities of utilizing oligosaccharides, polysaccharides, and glycoproteins and other glyco-conjugates for diagnosis, vaccine development, as new drug entities, and many other medical applications. Unfortunately, these potential applications are all impeded by the lack of large scale synthesis technology for these molecules. Metabolic engineering, since its inception in late 80’s, has grown to be a field impactful in the synthesis of a variety of molecules of commercial and societal importance. Opportunities abound at the interface of glycosciences and metabolic engineering. In fact, all sugar moieties in biological components, small or big, free or bound, are important targets for metabolic engineering. Over the past decades, its use in the synthesis of sugar-containing molecules has gained significance. Glycosidic bond formation catalyzed by glycosyltransferase enzyme is in the center of the synthesis of most glycan structures in nature. Oligosaccharides, polysaccharides and glycoproteins share the commonality that requires glycosyltransferases in their synthesis, differing only in the nature of the acceptors. Therefore, from a metabolic engineering point of view, they share much of the synthesis challenges. These include the high energy demand due to the need for sugar nucleotides as precursors, the complexity of metabolic pathways and regulations involved, and the adequate supply of acceptors when and where the glycosyltransferases are most active. Represented by 2’-fucosyllactose, the success in bringing highly valuable oligosaccharides to commercial production demonstrates the power of metabolic engineering. On the other hand, given the enormous diversity and significant complexity of saccharide-containing structures, a handful of molecules attaining commercial success can only qualify as a promising beginning. In fact, the surface of the gigantic glyco-sphere has barely scratched. Providing scientists with hundreds and thousands of glycans in quantities sufficient to probe their structure and function relationships and supplying clinicians with selective compounds (such as Globo H and heparin in Kg quantities) for clinical studies in a cost effective manner are challenges before metabolic engineers and synthetic biologists. The inherent challenges in complex carbohydrates demands innovative metabolic engineering strategies beyond a simple extension of those used in successful examples. In this presentation, metabolic engineering challenges common to glycosyltransferase-catalyzed synthesis of oligosaccharides are analyzed and successful examples from Chen labs are showcased to emphasize the power of metabolic engineering as an enabling technology.
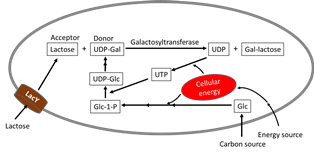
Reference:
- Hyun-Dong Shin, Long Liu, Mi-Kyong Kim, Yong-IL Park, Rachel Chen (2016) Metabolic engineering of Agrobacterium spp. ATCC31749 for curdlan production from cellobiose. J. Ind. Microbiol. Biotechnol. 43: 1323-1331.
- Anne Ruffing and Rachel Chen (2011) Citrate stimulates oligosaccharides synthesis in metabolically engineered Agrobacterium spp. Applied Biochemistry and Biotechnology 164(6):851-66.
- Mao Z, Shin H D, Chen R (2009) A recombinant E. coli bioprocess for hyaluronan synthesis. Applied Microbiology and Biotechnology 84:63-9.
- Anne Ruffing and Rachel Chen (2010) Metabolic engineering of Agrobacterium spp. strain ATCC31749 for production of an alpha-Gal epitope synthesis. Microbial Cell Factories 9:1.
- Anne Ruffing, Zichao Mao, Rachel Chen, (2006) Metabolic engineering of Agrobacterium spp. for UDP-galactose regeneration and oligosaccharide synthesis. Metabolic Engineering 8:465-473.
