Biography
Biography: Toshiyuki Moriuchi
Abstract
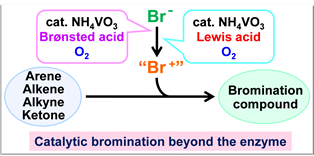
Haloperoxidases are enzymes that are able to catalyze the oxidation of halide ions by using hydrogen peroxide. Catalytic activities of haloperoxidases have received great attention because of their capability to halogenate a variety of organic compounds. Vanadium bromoperoxidase (V-BrPO), which is a naturally occurring enzyme found in marine algae, is a kind of haloperoxidase. V-BrPO catalyzes two-electron oxidation of the bromide ion in the presence of hydrogen peroxide, affording a bromonium cation-like species. V-BrPO has been demonstrated to perform the catalytic bromination of organic compounds. Bromination reaction is one of the most fundamental reactions in organic synthesis, providing important precursors and substrates in various coupling reactions. Conventional bromination reaction is performed by using hazardous and toxic elemental bromine. Considerable efforts have been focused on developing a versatile bromination method with a bromide ion as a bromide source instead of bromine. So, the V-BrPO mimicking bromination reaction systems induced by a vanadium catalyst and hydrogen peroxide have attracted much attention. These catalytic systems, however, require a stoichiometric amount of a strong oxidant to generate the bromonium-like species. A more practical catalytic bromination reaction system without use of hazardous reagents is to be developed. From the view point of green chemistry perspective, molecular oxygen is regarded as the best candidate for oxidants. We embarked upon the development of an environmentally-favorable catalytic method for selective bromination of a wide range of substrates. In this presentation, bromoperoxidase mimicking versatile and practical bromination catalytic systems by the combination of a commercially available inexpensive ligand-free vanadium catalyst and a Brønsted acid or a Lewis acid under molecular oxygen will be described.