
Claudio Santi
University of Perugia, Italy
Title: New drugs and catalysts inspired by glutathione peroxidase
Biography
Biography: Claudio Santi
Abstract
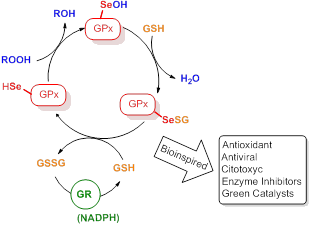
Glutathione Peroxidase (GPx) among the currently known selenoenzymes, is the best characterized in term of chemical structure and reaction mechanism. The catalytic center of this enzyme is a selenocysteine and, more specifically, a selenium atom that is stabilized by a catalytic triad in the form of nucleophilic selenate. In this form the selenium is reactive toward peroxides determining their reduction into the non-harmful alcohol or water. The selenol by reaction with the peroxide is transformed into the corresponding selenenic acid that is rapidly reduced by 2 molecules of glutathione affording a molecule of oxidized glutathione and the native selenate that is ready to start a second cycle. Glutathione peroxidase have a crucial role into control and prevent the damage produced by the reactive oxygen species (ROS) in living system and, from one side it is important to maintain a healthy status from the other it is necessary to reinforce it during a number of pathologic situation. During the last decades, several small molecules containing selenium as well as some artificial selenoenzymes were developed and tested as antioxidant but also as prooxidant as enzyme inhibitors, hormetic agents, antiviral, anticancer, antimicrobial. In this talk I’ll report the state of art of the research on this field focusing some new prospective that are currently ongoing in our laboratory: discovery of new biologically active organoselenium compounds and determination of their reaction mechanism in living systems. Beside that the bio inspiration is an excellent strategy for the development of new efficient and eco sustainable catalyst for application in Green Chemistry, some recent example of these reults will be presented and discussed.
References:
- Sancineto, L., Piccioni, M., De Marco, S., Pagiotti, R., Nascimento, V., Braga, A.L., Santi, C., Pietrella, D. Diphenyl diselenide derivatives inhibit microbial biofilm formation involved in wound infection (2016) BMC Microbiology, 16 (1), art. no. 220H
- Sancineto, L., Mariotti, A., Bagnoli, L., Marini, F., Desantis, J., Iraci, N., Santi, C., Pannecouque, C., Tabarrini, O. Design and Synthesis of DiselenoBisBenzamides (DISeBAs) as Nucleocapsid Protein 7 (NCp7) Inhibitors with anti-HIV Activity (2015) Journal of Medicinal Chemistry, 58 (24), pp. 9601-9614
- Bartolini, D., Commodi, J., Piroddi, M., Incipini, L., Sancineto, L., Santi, C., Galli, F. Glutathione S-transferase pi expression regulates the Nrf2-dependent response to hormetic diselenides (2015) Free Radical Biology and Medicine, 88 (Part B), pp. 466-480.
- Bartolini, D., Piroddi, M., Tidei, C., Giovagnoli, S., Pietrella, D., Manevich, Y., Tew, K.D., Giustarini, D., Rossi, R., Townsend, D.M., Santi, C., Galli, F. Reaction kinetics and targeting to cellular glutathione S-transferase of the glutathione peroxidase mimetic PhSeZnCl and its d,l-polylactide microparticle formulation (2015) Free Radical Biology and Medicine, 78, pp. 56-65.
- Santoro, S., Azeredo, J.B., Nascimento, V., Sancineto, L., Braga, A.L., Santi, C. The green side of the moon: Ecofriendly aspects of organoselenium chemistry (2014) RSC Advances, 4 (60), pp. 31521-31535..
